ESTABLISHMENT OF SEROTYPE-SPECIFIC IMMUNOLOGICAL MEMORY BY DIFFERENT INFANT IMMUNIZATION SCHEDULES WITH PCV13 (ID 39)
INVASIVE PNEUMOCOCCAL DISEASE (IPD) TRENDS 1998 TO MID-2019 IN CALGARY, CANADA: AN INTERRUPTED TIME SERIES ANALYSIS: A CASPER STUDY (ID 104)
PAEDIATRICS MENINGITIS SURVEILLANCE IN CÔTE D’IVOIRE AND USE OF IB-VPD/PBM TO MONITOR PCV IMPACT (ID 201)
- Cho n'din catherine Boni, Côte d'Ivoire
- Alice Britoh, Côte d'Ivoire
- Flore Zaba, Côte d'Ivoire
- Lepri Aka, Côte d'Ivoire
- Rebecca Kouamé, Côte d'Ivoire
- Hamidou Koné, Côte d'Ivoire
- Koffi Nzué, Côte d'Ivoire
- Rowan Bancroft, Côte d'Ivoire
- Jason M. M. Mwenda, Congo
- Brenda Kwambana, Côte d'Ivoire
- Antonio Martin, Côte d'Ivoire
Abstract
Background
National paediatric bacterial meningitis (PBM) surveillance began in 2002 in Côte d’Ivoire. This surveillance is under the supervision of the immunization program. The aim of this work was to describe the PBM surveillance in Côte d’Ivoire and use of IB-VPD/PBM to monitor PCV impact.
Methods
The paediatric service notifies suspected cases, collect and send the CSF samples to the laboratory. The laboratory receives and analyse CSF and do the management of data. The technical, logistical and financial support were provided by WHO, CDC and Regional Laboratory of GAMBIA MRC. The national immunization program provided feedback to central level.
Results
From January 2002 to December 2019, 7769 CSF samples were submitted to the sentinel site laboratory. Of these samples, 263(3.39%) gave a positive culture with bacterial growth. S. pneumoniae, 50.57% (133 /263) H. influenzae 30.08 (87 /263) and 5.7 % (15/263) N. meningitidies over all the years of monitoring. Pneumococcal conjugate vaccine (PCV) serotypes, 5, 18C, 19F and 6A/B were identified post-vaccine introduction.
Conclusions
This surveillance generated data used for to evidence of disease burden, and advocacy to introduce in routine immunization Pneumococcal conjugate vaccine -13 (PCV 13) in 2014.
EFFECTIVENESS OF PCV13 AGAINST INVASIVE PNEUMOCOCCAL DISEASE (IPD) AMONG ELDERLY UNDER INDIRECT EFFECTS FROM A MATURE INFANT PCV10 VACCINATION PROGRAMME (ID 235)
TWENTY YEAR IMPACT OF PNEUMOCOCCAL CONJUGATE VACCINE ON INVASIVE PNEUMOCOCCAL DISEASE SYNDROMES IN US CHILDREN LESS THAN 5 YEARS OF AGE (ID 243)
- Ruth Chapman, United Kingdom
- Kelly Sutton, United Kingdom
- Rotem Lapidot, United States of America
- Erica Chilson, United States of America
- Vincenza Snow, United States of America
- Shreeya Patel, United Kingdom
- Des Dillon-Murphy, United Kingdom
- Raymond A. Farkouh, United States of America
- Margaret Moffatt, United States of America
- Matt Wasserman, United States of America
- Stephen I. Pelton, United States of America
CHANGES IN ANTIMICROBIAL SUSCEPTIBILITY OF STREPTOCOCCUS PNEUMONIAE AMONG CHILDREN UNDER-FIVE YEARS ADMITTED WITH BACTERIAL MENINGITIS IN YAOUNDÉ AFTER PNEUMOCOCCAL CONJUGATE VACCINE INTRODUCTION IN CAMEROON. (ID 291)
TEMPORARY SHIFT IN SEROTYPES DISTRIBUTION OF STREPTOCOCCUS PNEUMONIAE CAUSING INVASIVE DISEASE IN ARGENTINA. (2006-2018) (ID 304)
DECLINES IN PNEUMONIA MORTALITY FOLLOWING THE INTRODUCTION OF PNEUMOCOCCAL CONJUGATE VACCINES IN LATIN AMERICAN AND CARIBBEAN COUNTRIES (ID 322)
- Kayoko Shioda, United States of America
- Lucia H. De Oliveira, United States of America
- Maria T. Valenzuela, Chile
- Cara B. Janusz, United States of America
- Analía Rearte, Argentina
- Alyssa N. Sbarra, United States of America
- Joshua L. Warren, United States of America
- Christina M. Toscano, Brazil
- Daniel M. Weinberger, United States of America
PNEUMOCOCCAL CARRIAGE AND DENSITY IN MONGOLIAN CHILDREN WITH RADIOLOGICALLY-CONFIRMED PNEUMONIA AND HEALTHY CHILDREN FROM THE COMMUNITY. (ID 387)
- Monica L. Nation, Australia
- Cattram D. Nguyen, Australia
- Eileen M. Dunne, United States of America
- Casey L. Pell, Australia
- Jason Hinds, United Kingdom
- Mukhchuluun Ulziibayar, Mongolia
- Bujinlkham Suuri, Mongolia
- Dashtseren Luvsantseren, Mongolia
- Tuya Mungun, Mongolia
- Kim E. Mulholland, Australia
- Claire Von Mollendorf, Australia
- Catherine Satzke, Australia
HIGH PNEUMOCOCCAL CARRIAGE RATES WITH DIVERSE SEROTYPES IN PAPUA NEW GUINEANCHILDRENWITH PNEUMONIA IN A RANDOMIZED TRIAL OF 10-VALENT AND 13-VALENT PNEUMOCOCCAL CONJUGATE VACCINES (ID 430)
- Mition J. Yoannes, Papua New Guinea
- Rebecca Ford, Papua New Guinea
- Audrey Michael, Papua New Guinea
- Vela Solomon, Papua New Guinea
- Geraldine Masiria, Papua New Guinea
- Gerard Saleu, Papua New Guinea
- Mary Dreyam, Papua New Guinea
- Birunu Nivio, Papua New Guinea
- Peter Siba, Papua New Guinea
- Peter Richmond, Australia
- Lea-Ann Kirkham, Australia
- Andrew R. Greenhill, Australia
- Deborah Lehmann, Australia
- William Pomat, Papua New Guinea
THE CHANGING EPIDEMIOLOGY OF INVASIVE PNEUMOCOCCAL DISEASE (IPD) IN ADULTS OF 65 YEARS OR OVER. IMPACT OF THE PAEDIATRIC PNEUMOCOCCAL VACCINATION PROGRAMME, SPAIN 2010-2017 (ID 470)
Abstract
Background
The paediatric heptavalent pneumococcal conjugate vaccine (PCV7) was first available in Spain in June 2001 and incorporated in the Madrid regional immunisation programme (RIP) in 2006, remaining in the private market for other regions. From mid-2010 through 2016, Spanish regions introduced the 13-valent conjugate vaccine (PCV13) in their RIPs. Adult vaccination with the 23-valent polysaccharide (PPV23) officially started in 2004, and with PCV13 in 2016 for some cohorts, without expected impact for this analysis.
Methods
Data source: cases reported through the European Centre for Disease Prevention and Control surveillance system (available online). IPD serotype specific counts were aggregated into PCV13-, PCV13 non-PCV7, 20-valent conjugate vaccine (PCV20) non-PCV13, PPV23-, and PPV23 non-PCV13-type groups. The percentage change in annual number of cases was estimated using linear regression analysis of the log of the annual number of cases.
Results
 During 2010-17, an 8.1% average annual decline (95%CI -13.7 to -2.2; p=0.02) in PCV13 non-PCV7-type IPD in adults was observed. Despite vaccination, PPV23 non-PCV13-type IPD increased an average 13.2% annually (95%CI 8.6 to 17.9; p<0.001).
During 2010-17, an 8.1% average annual decline (95%CI -13.7 to -2.2; p=0.02) in PCV13 non-PCV7-type IPD in adults was observed. Despite vaccination, PPV23 non-PCV13-type IPD increased an average 13.2% annually (95%CI 8.6 to 17.9; p<0.001).
Conclusions
Implementation of regional paediatric PCV13 programmes in Spain has been associated with decline of PCV13-types IPD in adults ≥ 65 years. However, further reductions may only be achieved with direct immunisation
EVOLUTION OF SEROTYPE DISTRIBUTION AND INCIDENCE RATES OF INVASIVE STREPTOCOCCUS PNEUMONIAE STRAINS IN CHILDREN (ID 491)
EFFECTIVENESS OF 13-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV13) TO PREVENT SEROTYPE 3 INVASIVE PNEUMOCOCCAL DISEASE (IPD) IN CHILDREN (ID 500)
THE IMPACT OF THE 10-VALENT PNEUMOCOCCAL CONJUGATE VACCINE ON INVASIVE DISEASE IN FIJI: A RETROSPECTIVE REVIEW (ID 504)
- Felista T. Ratu, Fiji
- Rita C. Reyburn, Australia
- Evelyn Tuivaga, Fiji
- Eileen M. Dunne, United States of America
- Devina Nand, Fiji
- Joseph Kado, Australia
- Lisi Tikoduadua, Fiji
- Kimberley Fox, Philippines
- Rachel Devi, Fiji
- Catherine Satzke, Australia
- Susan Ballard, Australia
- Eric Rafai, Fiji
- Kim E. Mulholland, Australia
- Stefan Flasche, United Kingdom
- Mike Kama, Fiji
- Fiona M. Russell, Australia
NASOPHARYNGEAL CARRIAGE PREVALENCE OF SEROTYPES 3, 6A, 19A AND 6C IN NEPALESE CHILDREN: IS IT TIME TO SWITCH FROM PCV10 TO A DIFFERENT PCV? (ID 528)
- Pratistha Maskey, Nepal
- Meeru Gurung, Nepal
- Sanjeev M. Bijukchhe, Nepal
- Peter J. O'Reilly, United Kingdom
- Himang M. Maskey, Nepal
- Subhash Shrestha, Nepal
- Stephen Thorson, Nepal
- Madhav C. Gautam, Nepal
- Bhishma Pokhrel, Nepal
- Ganesh Shah, Nepal
- Imran Ansari, Nepal
- Sarah Kelly, United Kingdom
- Merryn Voysey, United Kingdom
- Maria Deloria Knoll, United States of America
- Dominic Kelly, United Kingdom
- David Murdoch, New Zealand
- Andrew J. Pollard, United Kingdom
- Shrijana Shrestha, Nepal
COMMUNITY ONSET PNEUMONIA INCIDENCE IN ADULTS 18 YEARS AND OLDER IN GOTO ISLAND, JAPAN. INTERIM RESULTS FROM A POPULATION BASED PROSPECTIVE STUDY (ID 596)
- Taiga Miyazaki, Japan
- Katsuji Hirano, Japan
- Shigeru Kohno, Japan
- Kiyoshi Ichihara, Japan
- Elisa N. Gonzalez, United States of America
- Pingping Zhang, United States of America
- Raul Isturiz, United States of America
- Bradford D. Gessner, United States of America
- Luis Jodar, United States of America
- Adriano Arguedas, United States of America
S. PNEUMONIAE (SPN) CAUSING INVASIVE PNEUMOCOCCAL DISEASE (IPD) AMONG ADULTS IN ARGENTINA: THEORETICAL VACCINE COVERAGE (TVC) OF PCV-13 AND NEW CONJUGATED VACCINES (CVS) (ID 610)
EFFECTIVENESS OF PPV23 PNEUMOCOCCAL VACCINE AGAINST PNEUMOCOCCAL COMMUNITY ACQUIRED PNEUMONIA IN HOSPITALIZATION CANADIAN ADULTS (ID 617)
- May ElSherif, Canada
- Jason J. LeBlanc, Canada
- Lingyun Ye, Canada
- Donna MacKinnon-Cameron, Canada
- Ardith Ambrose, Canada
- Todd F. Hatchette, Canada
- Amanda L. Lang, Canada
- Hayley D. Gillis, Canada
- Irene Martin, Canada
- Walter Demczuk, Canada
- Craig LaFerriere, Canada
- Melissa K. Andrew, Canada
- Guy Boivin, Canada
- William Bowie, Canada
- Karen Green, Canada
- Jennie Johnstone, Canada
- Mark Loeb, Canada
- Anne E. McCarthy, Canada
- Allison McGeer, Canada
- Makeda Semret, Canada
- Sylvie Trottier, Canada
- Louis Valiquette, Canada
- Duncan Webster, Canada
- Shelly A. McNeil, Canada
REPLACEMENT DISEASE WITH PNEUMOCOCCAL CONJUGATE VACCINES: IMPACT ON OVERALL INVASIVE PNEUMOCOCCAL DISEASE IN CHILDREN AND OLDER ADULTS (ID 637)
SEROTYPES CAUSING INVASIVE PNEUMOCOCCAL DISEASE BEFORE AND AFTER PCV10 IN A BRAZILIAN HOSPITAL: A TWETY YEAR HOSPITAL-BASED SURVEILLANCE STUDY (ID 738)
DECLINE IN CHILD HOSPITALIZATION AND MORTALITY AFTER THE INTRODUCTION OF THE 7-VALENT PNEUMOCOCCAL CONJUGATIVE VACCINE (PCV-7) IN RWANDA (ID 808)
EFFECTIVENESS OF PNEUMOCOCCAL CONJUGATE VACCINES (PCVS) TO PREVENT SEROTYPE 19A INVASIVE PNEUMOCOCCAL DISEASE (IPD) IN CHILDREN (ID 835)
GLOBAL SEROTYPE DISTRIBUTION OF INVASIVE PNEUMOCOCCAL DISEASE (IPD) AMONG CHILDREN IN COUNTRIES WITH MATURE PCV10/13 PROGRAMS: THE PNEUMOCOCCAL SEROTYPE REPLACEMENT AND DISTRIBUTION ESTIMATION (PSERENADE) PROJECT (ID 883)
SEROTYPE 3 IS NOT A COMMON NASOPHARYNGEAL COLONIZER OR ACUTE OTITIS MEDIA PATHOGEN IN CHILDREN IN ROCHESTER, NY (ID 885)
ANTIBIOTIC RESISTANCE PATTERNS AND PHYLOGEOGRAPHY OF STREPTOCOCCUS PNEUMONIAE ASSOCIATED WITH PAEDIATRIC MENINGITIS IN PRE- AND POST-PCV-INTRODUCTION WEST AND CENTRAL AFRICA (ID 958)
- Madikay Senghore, United States of America
- Peggy-Estelle Tientcheu,
- Archibald Worwui,
- Sheikh Jarju,
- Catherine Okoi,
- Sambou Susso,
- Ebenezer Foster-Nyarko,
- Chinelo Ebruke,
- Mohamadou Sonko,
- Mamadou H. Korma,
- Joseph Agossou,
- Enyonam Tsolenyanu, Togo
- Lorna A. Renner,
- Daniel Ansong,
- Bakary Sanneh,
- Cho n'din catherine Boni, Côte d'Ivoire
- BOULA A. Yvette, Cameroon
- Berthe Miwanda,
- Stephanie Lo, United Kingdom
- Rebecca Gladstone, Norway
- Stephanie Schwartz,
- Paulina A. Hawkins, Brazil
- Lesley McGee, United States of America
- Keith P. Klugman, United States of America
- Robert F. Breiman, United States of America
- Stephen D. Bentley, United Kingdom
- Jason M. M. Mwenda, Congo
- Brenda Kwambana-Adams, United Kingdom
- Martin Antonio, Gambia
LONG TERM HOSPITAL-BASED SURVEILLANCE ON INVASIVE PNEUMOCOCCAL DISEASE: MOVING TOWARDS AN ADULT DISEASE? (ID 1074)
PNEUMOCOCCAL CARRIAGE IN CHILDREN WITH PNEUMONIA IN THREE ASIAN COUNTRIES FOLLOWING VACCINE INTRODUCTION (ID 1082)
- Catherine Satzke, Australia
- Eileen M. Dunne, United States of America
- Jocelyn Chan,
- Monica L. Nation, Australia
- Keoudomphone Vilivong, Laos
- Belinda D. Ortika, Australia
- Mimee Laddaphone, Laos
- Rebecca Ford, Papua New Guinea
- Joycelyn J. Sapura, Papua New Guinea
- John Kave, Papua New Guinea
- Cattram D. Nguyen, Australia
- Casey L. Pell, Australia
- Ahmed Alamrousi, Australia
- Jason Hinds, United Kingdom
- Paul N. Newton, United Kingdom
- Anonh Xeuatvongsa, Laos
- B Bunjinlham,
- Christopher C. Blyth, Australia
- David A. Dance, Laos
- William Pomat, Papua New Guinea
- Claire Von Mollendorf, Australia
- Tuya Mungun, Mongolia
- Kim E. Mulholland, Australia
- Fiona M. Russell, Australia
CHANGE OF S. PNEUMONIAE SEROTYPES CAUSING MENINGITIS AND ANTIMICROBIAL SUSCEPTIBILITY IN CHILDREN POPULATION AFTER INTRODUCTION OF PNEUMOCOCCAL CONJUGATE VACCINES (ID 1094)
TRENDS IN INVASIVE PNEUMOCOCCAL DISEASE (IPD) IN ADULTS OF 65 YEARS OR OVER, ITALY, 2012-2017 (ID 1184)
Abstract
Background
The paediatric heptavalent pneumococcal conjugate vaccine (PCV7) was licensed in 2001 and added to the National Immunisation Programme (NIP) in 2005. The 13-valent conjugate vaccine (PCV13) entered the paediatric NIP in 2012. PCV13 adult vaccination started in 2012 for at-risk population in some regions and introduced in NIP in 2017 sequentially with the 23-valent polysaccharide (PPV23), used before in at-risk and older people.
Methods
Cases were reported through the European Centre for Disease Prevention and Control surveillance system. IPD serotype specific counts were aggregated into PCV13-, PCV13 non-PCV7-, 20-valent conjugate vaccine (PCV20) non-PCV13-, PPV23-, and PPV23 non-PCV13-type groups. The percentage change in annual number of cases was estimated using linear regression analysis of the log of annual number of cases.
Results
During 2012-17, there was an average annual increase of 34% for PCV20 non-PCV13- (95%CI 20.4 to 48.8; p=0.002), of 16% for PPV23- (95%CI 7.5 to 25.1; p=0.006), and of 31.5% for PPV23 non-PCV13- (95%CI 18.1 to 46.4; p=0.002)-, without significant changes in PCV13-, PCV13 non-PCV7-type groups
Conclusions

Implementation of the paediatric PCV13 programme, despite high coverage, does not appear to be associated with changes in PCV13-type IPD in adults. Reductions may be achieved with direct immunisation with PCV13 or next-generation vaccine (PCV20)
IMPACT OF THE PNEUMOCOCCAL CONJUGATE VACCINE ON PNEUMONIA MORTALITY IN SOUTH AFRICA, 1999-2016: A RETROSPECTIVE OBSERVATIONAL STUDY (ID 118)
INCREASE IN PCV13-ONLY SEROTYPE INVASIVE PNEUMOCOCCAL DISEASE IN CHILDREN AND OLDER PERSONS DURING PCV10 PERIOD (2015-2019) IN BELGIUM (ID 252)
HEALTH-AUGMENTED LIFECYCLE-MODEL-BASED ECONOMIC EVALUATION OF PCV13 PEDIATRIC IN INDONESIA, MALAYSIA, AND THAILAND (ID 271)
- JP Sevilla, United States of America
- Daria Burnes, United States of America
- Pichaya Suthipinijtham, Thailand
- Carolina Halim, Indonesia
- Jerusha Naidoo, Malaysia
- Matt Wasserman, United States of America
- Sarah Pugh, United States of America
- Asrul Shaife, Malaysia
- Soewarta Kosen, Indonesia
- Nathorn Chaiyakunapruk, United States of America
- David Bloom, United States of America
TRENDS IN LABORATORY-CONFIRMED PNEUMOCOCCAL MENINGITIS AFTER INTRODUCTION OF 13-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV13) INTO THE NATIONAL IMMUNIZATION PROGRAMME IN CAMEROON (ID 299)
INVASIVE PNEUMOCOCCAL DISEASE IN CHILDREN: CLINICAL DISCREPANCIES BETWEEN VACCINE AND NON-VACCINE SEROTYPES (ID 300)
Abstract
Background
Following introduction of the 13-valent pneumococcal conjugate vaccine (PCV13), concern regarding protection against invasive pneumococcal disease (IPD) caused by serotypes (ST) 3 and, to a lesser degree, 19A has been raised. We hypothesized that PCV13 STs, specifically ST3, are strongly associated with respiratory (R)-IPD and rare in non-respiratory (NR)-IPD.
Methods
Clinical and isolate details from a prospective surveillance study of pediatric IPD in Orange County, CA (2010-2019) are presented. R-IPD and NR-IPD group differences were assessed using the Chi-square test.
Results
Serotype was available for 181 IPD cases, of which 37% were PCV13-STs (54% of 78 R-IPD, 24% of 103 NR-IPD, p<.001). Of these, 32 (48%) had received no PCV13 immunization with 26 (81%) having occurred during first half of study. ST3 (21% and 4%, p<.001) and ST19A (21% and 8%, p=.01) were recovered from R-IPD and NR-IPD cases, respectively. Combined ST3 and ST19A accounted for 76% of PCV13 isolates in R-IPD and 48% in NR-IPD, p=.02. Most PCV13-STs occurred during fall-winter (63%).
Conclusions
R-IPD was predominantly associated with PCV13-STs, particularly ST3 and ST19A. PCV13 R-IPD was more common during fall-winter. The role of winter respiratory viruses in nasopharyngeal colonization with PCV13-STs and the timing of booster PCV13 dosing should be further researched.
SHIFTING EPIDEMIOLOGY OF PNEUMOCOCCAL VACCINE SEROTYPES AMONG VARIOUS AGE GROUPS IN CANADA FROM 2010 TO 2018 (ID 337)
PCV15 AND PPSV23 COVERAGE OF INVASIVE AND RESPIRATORY TRACT ISOLATES OF STREPTOCOCCUS PNEUMONIAE: CANWARD 2007-2018 (ID 346)
Abstract
Background
The objective of this study was to compare the proportion of invasive and respiratory tract isolates of Streptococcus pneumoniae (SPN), including multidrug/extensive-drug resistant (MDR/XDR) strains, that demonstrated PCV15/PPSV23 serotypes in Canada from 2007-2018.
Methods
The CANWARD study collected 2821 SPN isolates from 2007-2018 (986 invasive, 1835 respiratory). Serotyping was performed by the Quellung reaction. Antimicrobial susceptibility testing was performed using CLSI methods. MDR/XDR was defined as resistance to ³3/³5 antimicrobial classes, respectively.
Results
Overall, the proportion of blood isolates demonstrating a PCV15/PPSV23 serotype was significantly higher than respiratory strains (55.4/76.7% vs 39.5/55.3%, P<0.0001). By age group, with the exception of the <2-year category, the proportion of blood isolates demonstrating a PCV15/PPSV23 serotype was significantly higher than respiratory strains (P</=0.0046). Similar results were noted by gender (P<0.0001) and region (P</=0.0002), with the exception of Eastern Canada. There was no significant difference in the proportion of MDR blood (64.4/71.1%) and respiratory (54.3/56.1%) isolates representing PCV15/PPSV23 serotypes, respectively. All XDR isolates were serotypes contained in PCV15/PPSV23, and there was no significant difference in proportion between blood (92.9%) and respiratory (88.9%) isolates.
Conclusions
In general, the proportion of blood isolates demonstrating a PCV15/PPSV23 serotype in Canada was significantly higher than that of respiratory isolates.
ANTIMICROBIAL SUSCEPTIBILITY OF PNEUMOCOCCAL CARRIAGE ISOLATES AMONG PAPUA NEW GUINEAN CHILDREN VACCINATED WITH 10-VALENT OR 13-VALENT PNEUMOCOCCAL CONJUGATE VACCINES (PCVS) (ID 388)
- Tilda Orami, Papua New Guinea
- Audrey Michael, Papua New Guinea
- Rebecca Ford, Papua New Guinea
- Mition J. Yoannes, Papua New Guinea
- Vela Solomon, Papua New Guinea
- Gerard Saleu, Papua New Guinea
- Geraldine Masiria, Papua New Guinea
- Mary Dreyam, Papua New Guinea
- Birunu Nivio, Papua New Guinea
- Anita Van Den Biggelaar, Australia
- Lea-Ann Kirkham, Australia
- Andrew R. Greenhill, Australia
- Peter Richmond, Australia
- Deborah Lehmann, Australia
- Peter Siba, Papua New Guinea
- William Pomat, Papua New Guinea
TEMPORAL CHANGES IN PNEUMOCOCCAL COLONIZATION IN UNDER-5 YEAR OLD CHILDREN EIGHT YEARS FOLLOWING ROUTINE INFANT IMMUNIZATION WITH PNEUMOCOCCAL CONJUGATE VACCINE (PCV) IN RURAL SOUTH AFRICA (ID 413)
PNEUMOCOCCAL CARRIAGE PRE- AND POST- PCV: A SYSTEMATIC REVIEW (ID 431)
- Nicole Wong, Australia
- Eleanor F. Neal, Australia
- Sam Clifford, United Kingdom
- Belinda D. Ortika, Australia
- Kyla Hayford, United States of America
- Shereen Labib, Australia
- Julia Bennett, United States of America
- Maria D. Knoll, United States of America
- Stefan Flasche, United Kingdom
- Fiona M. Russell, Australia
PNEUMOCOCCAL DISEASE PREVENTION: MORE THAN JUST COUNTING SEROTYPES? (ID 527)
IMPACT OF PCV10 IN NEPAL ON NASOPHARYNGEAL CARRIAGE OF PNEUMOCOCCUS IN YOUNG INFANTS PRIOR TO THEIR VACCINATION (ID 530)
- Subhash Shrestha, Nepal
- Meeru Gurung, Nepal
- Brian Wahl, United States of America
- Sanjeev M. Bijukchhe, Nepal
- Peter J. O'Reilly, United Kingdom
- Pratistha Maskey, Nepal
- Himang M. Maskey, Nepal
- Madhav C. Gautam, Nepal
- Stephen Thorson, Nepal
- Bhishma Pokhrel, Nepal
- Ganesh Shah, Nepal
- Imran Ansari, Nepal
- Sarah Kelly, United Kingdom
- Merryn Voysey, United Kingdom
- Maria D. Knoll, United States of America
- Dominic Kelly, United Kingdom
- David Murdoch, New Zealand
- Andrew J. Pollard, United Kingdom
- Shrijana Shrestha, Nepal
IMPACT OF THE INTRODUCTION OF THE PNEUMOCOCCAL CONJUGATE VACCINE ON PAEDIATRIC PNEUMONIA CASES IN NEPAL (ID 543)
- Ganesh Shah, Nepal
- Meeru Gurung, Nepal
- Merryn Voysey, United Kingdom
- Sanjeev M. Bijukchhe, Nepal
- Peter J. O'Reilly, United Kingdom
- Stephen Thorson, Nepal
- Animesh K. Basnet, Nepal
- Sunaina Gurung, Nepal
- Bhishma Pokhrel, Nepal
- Sarah Kelly, United Kingdom
- Maria D. Knoll, United States of America
- Dominic Kelly, United Kingdom
- David Murdoch, New Zealand
- Andrew J. Pollard, United Kingdom
- Shrijana Shrestha, Nepal
SEVEN YEARS EXPERIENCE USING PNEUMOCOCCAL CONJUGATE VACCINES IN CHILE: WHAT HAVE WE LEARNED? (ID 547)
SEROTYPE DISTRIBUTION OF PNEUMOCOCCI ISOLATED FROM CHILDREN WITH RESPIRATION INFECTION FROM 2017 TO 2019 IN SUZHOU, CHINA (ID 568)
ASSOCIATION OF ROUTINE INFANT 2+1 13-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV) WITH STREPTOCOCCUS PNEUMONIAE CARRIAGE AND SEROTYPE EPIDEMIOLOGY IN URBAN-DWELLING SOUTH AFRICAN CHILDREN AGED ≤5YEARS. (ID 590)
NUMBER OF CHILDREN WITHOUT 13-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV13) SERIES COMPLETION AT 2 YEARS OF AGE IN CANADA (ID 591)
Abstract
Background
Despite more than 6 years of routine pediatric PCV13 implementation in Canada, PCV13 serotypes still contributed to roughly 25% of invasive pneumococcal disease in older adults in 2017. One of the elements that can affect disease transmission and immunization program effectiveness is vaccine schedule completion. With transition to PCV13 in 2010/2011, a reduced, 2+1 routine immunization schedule was adopted across Canadian provinces, with the third dose recommended at 12 months of age.
Methods
We estimated the cumulative number of children with incomplete routine PCV13 vaccination series from 2011 to 2017, measured at 2 years of age. National Immunization Survey Coverage rates (available for 2011, 2013, 2015 and 2017; averaged for 2012, 2014 and 2016) along with Canadian census data were used to derive the number of children with incomplete PCV13 series.
Results
There were ~609,000 children with incomplete PCV13 series at 2 years of age in Canada in 2011-2017 (Table 1).
Conclusions
The substantial estimated number of children with incomplete PCV13 series by 2 years of age over the study period, coupled with reduced-dose schedule may have undermined achievement of optimal public health impact. Potential role of these factors in PCV13 program effectiveness in Canada requires better understanding.
PNEUMOCOCCAL COLONIZATION EPIDEMIOLOGY IN RURAL-DWELLING PRIMARY-CARE-GIVERS OF CHILDREN ≤5YEARS OF AGE, 8 YEARS FOLLOWING ROUTINE PNEUMOCOCCAL CONJUGATE VACCINE (PCV) IMMUNIZATION OF SOUTH AFRICAN INFANTS. (ID 597)
HOSPITALIZATIONS FOR LOWER RESPIRATORY TRACT INFECTIONS IN CHILDREN IN RELATION TO THE SEQUENTIAL USE OF THREE PNEUMOCOCCAL VACCINES IN QUEBEC (ID 598)
POST-VACCINATION EPIDEMIOLOGY OF PNEUMOCOCCAL CARRIAGE AMONG CHILDREN YOUNGER THAN FIVE YEARS OF AGE IN CAPE COAST, GHANA (ID 685)
IS SEROTYPE DISTRIBUTION SIMILAR IN INVASIVE AND NON-INVASIVE PNEUMOCOCCAL DISEASE AND CAN IT PREDICT THE FULL IMPACT OF PNEUMOCOCCAL CONJUGATE VACCINES? (ID 711)
DISEASE FEATURES OF VIETNAMMESE INFANTS FROM HOSPITAL ADMISSIONS OF A PNEUMOCOCCAL VACCINE PHASE 3 CLINICAL TRIAL (ID 741)
ANTIMICROBIAL RESISTANCE IN THE PNEUMOCOCCAL CONJUGATE VACCINES ERA: WHAT HAVE WE LEARNED? A SYSTEMATIC LITERATURE REVIEW (ID 766)
LACK OF IMPACT OF PNEUMOCOCCAL CONJUGATE VACCINATION ON SEROTYPE-SPECIFIC PNEUMOCOCCAL CARRIAGE DENSITY IN PAPUA NEW GUINEAN INFANTS (ID 769)
- Kate Britton, Australia
- Janessa Pickering, Australia
- William Pomat, Papua New Guinea
- Camilla DeGier, Australia
- Monica L. Nation, Australia
- Casey L. Pell, Australia
- Caitlyn Granland, Australia
- Vela Solomon, Papua New Guinea
- Andrew R. Greenhill, Australia
- Peter Richmond, Australia
- Christopher C. Blyth, Australia
- Deborah Lehmann, Australia
- Catherine Satzke, Australia
- Lea-Ann Kirkham, Australia
THE POTENTIAL PROTECTION OFFERED BY BROADER PCV VACCINES IN OLDER ADULTS – IRISH IPD SURVEILLANCE FROM 2007-08 TO 2018-19 (ID 810)
EFFECTIVENESS OF 23-VALENT PNEUMOCOCCAL POLYSACCHARIDE VACCINE (PPV-23) IN THE PREVENTION OF HOSPITALIZATIONS DUE TO VACCINE-TYPE PNEUMOCOCCAL PNEUMONIA (ID 811)
- Julio Ramirez, United States of America
- Stephen Furmanek, United States of America
- Senen Pena, United States of America
- William Mattingly, United States of America
- Forest Arnold, United States of America
- Tim Wiemken, United States of America
- Ruth Carrico, United States of America
- The Louisville Pneumonia Study Group, United States of America
CLONAL COMPLEXES AND SEQUENCE TYPES OF STREPTOCOCCUS PNEUMONIAE SEROTYPE 19A CIRCULATING IN THE PRE- AND POST-VACCINE ERA: A SYSTEMATIC LITERATURE REVIEW (ID 813)
FIVE YEARS OF PNEUMONIA SURVEILLANCE IN LAO PDR (ID 925)
- Laddaphone Bounvilay, Laos
- Keoudomphone Vilivong,
- Jana Y. Lai, Australia
- Jocelyn Chan,
- Eileen M. Dunne, United States of America
- Kimberly Fox,
- Paul N. Newton, United Kingdom
- Mayfong Mayxay, United Kingdom
- Anonh Xeuatvongsa, Laos
- Kim E. Mulholland, Australia
- Catherine Satzke, Australia
- Audrey Dubot-Pérès, Laos
- David A. Dance, Laos
- Fiona M. Russell, Australia
Abstract
Background
Laos has one of the highest under-five mortality rates in South East Asia, with pneumonia being a leading cause. Hospital-based sentinel site pneumonia surveillance was established at the main tertiary referral hospital in the capital city, Vientiane. We describe the epidemiology of paediatric pneumonia and the detection of potential pathogens from upper respiratory tract samples since PCV13 introduction in 2013.
Methods
From 2013-2019, we enrolled children aged 2-59 months admitted with acute respiratory infection. Oral, throat and nasopharyngeal swabs were taken. Clinical and socioeconomic details were recorded. PCV13 status was recorded from written records. Pneumonia was classified according to the WHO 2013 definition. Multiplex PCR was used to detect respiratory viruses. Pneumococci were detected using lytA qPCR and serotyped using microarray.
Results
1436 were enrolled, of whom 859 had pneumonia. The median age of pneumonia cases were 15 months (IQR 6-21 months), 53.5% had severe pneumonia, 33.5% were hypoxic, and 1.8% died or were discharged unwell. Malnutrition was present in 5.6%. RSV was seasonal and common in young children. PCV13-type carriage declined in vaccinated and under-vaccinated cases.
Conclusions
Childhood pneumonia is a common reason for hospital admission in Laos. There is some evidence of direct and indirect effects of PCV13. RSV is common.
CHANGES IN SEROTYPE (ST) DISTRIBUTION OF INVASIVE PNEUMOCOCCUS PNEUMONIAE STRAINS IN (ID 982)
IN-DEPTH ANALYSIS OF PNEUMOCOCCAL SEROTYPES IN BELGIAN CHILDREN (2015-2018): DIVERSITY AND INVASIVE DISEASE POTENTIAL OF SEROTYPES IN CARRIAGE AND DISEASE (ID 1032)
Abstract
Background
Over a three year period (2015-2018) during and immediately after the PCV13-to-PCV10-switch in Belgium, nasopharyngeal pneumococcal carriage monitoring and IPD surveillance data are used to study invasive disease potential and distribution of pneumococcal serotypes.
Methods
Strains isolated from children up to 30 months of age, either attending day-care or diagnosed with IPD were serotyped by Quellung-reaction. Invasive disease potential was defined as the serotype-specific Odds ratio (OR).
Results
Over the entire study period, different serotypes dominated in each population: 12F, 19A, 10A, 33F in IPD versus 23B, 23A, 11A, 15B in carriage, but the proportion of non-PCV13 vaccine serotypes (VT) was high in both (IPD: 83.0%; carriage: 93.6%). The highly invasive (OR>1) serotypes (12F, 1, 3, 24A/B/F, 33F, 19A, 9N) accounted for 53.2% of IPD cases and were not frequently carried (<3.5%). From 2015 to 2018, PCV13 vaccine serotypes increased (p<0.01) in carriage (from 5.4%, 25/463 to 10.3%, 69/668) and in IPD-surveillance (from 7.3%, 8/110 to 23.9%, 34/142) mainly due to an increase (p<0.01) in serotype 19A.

Conclusions
A majority of the serotypes with high invasive disease potential circulating in Belgium are not included in the currently used PCVs, reinforcing the need for continuous IPD surveillance and carriage monitoring.
DELINEATING THE PERTURBATION BY PCV13 IN COMPOSITION OF STREPTOCOCCUS PNEUMONIAE CARRIAGE ISOLATES IN CAMBODIA (ID 1159)
Abstract
Background
We sought to elucidate the perturbation by PCV13 to the Streptococcus pneumoniae strain and serotype composition in Cambodian carriage isolates.
Methods
Pre-PCV13 (01/2013–12/2015, N=258) and the post-PCV13 isolates (01/2016-02/2017, N=432) were sequenced and analysed using PopPUNK(https://github.com/johnlees/PopPUNK) and SeroBA (https://github.com/sanger-pathogens/seroba) to determine strain prevalence and serotype composition.
Results
PCV13 serotypes significantly decreased by Fisher’s exact test (p=0.003[95% Confidence interval 0.45-0.85], OR 0.62) while non-PCV13 serotype significantly increased(p=0.002[1.18-2.26], OR 1.64) in the post-PCV13 populations. There was a significant increase in Simpsons diversity index for both serotype (Welch’s t-test p=0.0059) and strain (p=0.0228) in the post PCV13 population. The isolates were comprised of 44 unique serotypes with 27 pre-PCV and 32 post-PCV13. Significant changes in prevalence were detected in the post-PCV13 populations of serotypes 19F (N=52, 98.1% GPSC1; p=0.02[0.26-0.89], OR 0.48), 23A (N=27, 96.3% GPSC626; p=0.03 [1.04-9.69], OR 2.84), 34 (N=25, 100% GPSC45; p=0.01 [1.35-24], OR 4.55), and 6D (N=8, 87.5% GPSC16; p=0.03[1.19-Inf], OR Inf).
Conclusions
The strain population in Cambodia has been perturbed by the vaccine but had not yet reached equilibrium 24 months following PCV13 introduction. Additional isolate collection is ongoing for detection of trends towards equilibrium post-PCV13 in this population.
REDUCTION IN PNEUMOCOCCAL NASOPHARYNGEAL CARRIAGE WITH THREE DIFFERENT SCHEDULES OF PNEUMOCOCCAL CONJUGATE VACCINES IN INFANCY (ID 1193)
FOLLOWING A DECADE OF PCV IN THE GAMBIA SHOULD A DECLINE IN RESISTANCE BE ANTICIPATED? (ID 1205)
- Muhammed Arafat Cham, Gambia
- Brenda Kwambana-Adams, United Kingdom
- Madikay Senghore, United States of America
- Effua Usuf, Gambia
- Archibald Worwui,
- Rasheed Salaudeen, Gambia
- Lesley McGee, United States of America
- Stephen D. Bentley, United Kingdom
- Robert F. Breiman, United States of America
- Anna Roca, Gambia
- Grant Mackenzie, Gambia
- Martin Antonio, Gambia
CANADIAN ADULTS 50-64 YEARS OF AGE CONTRIBUTE SUBSTANTIALLY TO THE CASES OF INVASIVE PNEUMOCOCCAL DISEASE (IPD) POTENTIALLY PREVENTABLE BY THE 13-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (ID 1230)
Abstract
Background
In Canada, age-based recommendation for adult pneumococcal vaccination starts at 65 years (routine for PPV23, on an individual basis for PCV13). Recent literature reports large additional pneumococcal pneumonia and non-pneumonia IPD burden in Canadian adults aged 50-64 years.
Methods
Case counts of IPD by serotype and age group were obtained from published annual National Microbiology Laboratory (NML) reports of passive laboratory-based surveillance. We calculated the proportion of all IPD cases occurring in adults 50-64, ≥65, and ≥50 years of age and trends in the proportion of PCV13-type IPD in these age groups, for 2010-2017.
Results
Between 2010 and 2017, adults aged 50-64 and ≥65 contributed 27% and 38% of 21, 610 reported IPD cases, respectively. The proportion of PCV13-type IPD declined from 52% to 34% in 50-64 cohort, and 50% to 23% in ≥65 cohort, showing a plateau since 2014 in all three age groups (Figure 1).
Conclusions
Adults ≥65 years contributed 38%, and those 50-64 years an incremental 27% of all IPD in Canada over the study period. In 2017, 23% and 34% of IPD in these two cohorts, respectively, was of PCV13-type. These findings support the rationale for intensified PCV13 immunization efforts in both age groups.
SEROTYPE REPLACEMENT IN ENGLAND: THE BIAS OF INCREASED REPORTING (ID 1231)
Abstract
Background
Increased incidence of invasive pneumococcal disease (IPD) attributable to non-vaccine serotypes (NVT) has been reported in several countries following introduction of PCV7 and PCV13 vaccines, concurrently with a reduction in vaccine-type IPD. Such serotype replacement has, importantly, emerged in England, offsetting the benefit of PCV introduction. We scrutinise most recent findings to assess if the estimated increase in NVT disease might result from surveillance artefacts.
Methods
Using IPD surveillance for 2000-2018, we estimate the impact of PCV7 and PCV13 introduction on age-serotype-specific incidence rates through a synthetic control regression model, building counterfactuals by combining age-specific incidences reported for pathogens unaffected by PCVs.
Results
Following the introduction of PCV7 and PCV13 (pre-2006 vs post-2011), total IPD incidence declined by 57% and by 76% in children younger than 5. PCV7-IPD decreased by 93% in all age groups, whereas PCV13-IPD declined by 68% since PCV13 was introduced. Importantly, NVT-IPD increased by 43% after PCV7, with non-significant statistical increases in most age groups.
Conclusions
Through appropriate statistical modelling, we disentangled the impact of vaccine and improved surveillance on the changes in IPD incidence rates. By controlling for the confounding effects of improved surveillance, we are able to estimate a lower serotype replacement.
PREVALENCE OF PNEUMOCOCCUS SEROTYPES IN MENINGITIS AT THE MOTHER AND CHILD CENTRE AFTER INTRODUCTION OF PCV13 VACCINE IN CAMEROON BETWEEN 2013 TO 2018. (ID 33)
Abstract
Background
The major cause of morbidity and mortality in children below 5 years old, Streptococcus pneumoniae meningitis is a global scourge. Since 1 July 2011, the PCV13 pneumococcal vaccine has been introduced into the routine immunization program in Cameroon. We describe the serotypes that caused pneumococcal meningitis after introducing the vaccine.
Methods
Pneumococcal isolates of children below 5 years of age between January 2013 and December 2018 sentinel surveillance for meningitis conducted at the Mother and Child Center, were serotyped and sequentially multiplexed polymerase chain reaction.
Results
Of the 64 cases of confirmed streptococcal pneumoniae meningitis, 41 (64.06%) were analyzed for serotype identification and 34 serotypes were obtained. The most common are: 6A-6B-15B-2-4-5-12F-7A-7F-12A-12B-16F-17F-25F-44. The highest frequency of types 6A and 6B (14.71%) is noted. We observe a high frequency of serotypes 2 and 5 in the age group 0-5Month: 3/16 (18.75%). 2/14 (14.28%) of children in the age group 6-23Month have serotype 12A / 12B / 12F / 44/4. As for the age group of 24-59M, the most represented serotype is 15B: 2/11(18.18%).
Conclusions
Although the PCV13 vaccine is beneficial in Cameroon, it is necessary to constantly monitor the emergence of non-vaccinal serotypes because from the results, we note the emergence of other serotypes.
IMPACT OF 13-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV13) ON NON-BACTEREMIC PNEUMOCOCCAL PNEUMONIA (NBPP) IN THE UNITED STATES 2013-2017 (ID 222)
- Ryan Gierke, United States of America
- Almea Matanock,
- Nong Shang,
- Monica Farley, United States of America
- William Schaffner, United States of America
- Ann Thomas, United States of America
- Arthur Reingold, United States of America
- Lee Harrison, United States of America
- Katherine Schleiss,
- Kari Burzlaff, United States of America
- Susan Petit, United States of America
- Nisha Alden,
- Tamara Pilishvili, United States of America
Abstract
Background
PCV13 was recommended for U.S. children in 2010 and for adults ≥65 years in 2014. Vaccine coverage among adults ≥65 years was 43% in 2017. We evaluated PCV13 impact on NBPP among adults.
Methods
NBPP cases (clinically or radiographically-confirmed pneumonia and a positive pneumococcal urine antigen test in a hospitalized adult aged ≥18 years) were identified at select hospitals in 10 sites within CDC’s Active Bacterial Core surveillance during 2013-2017. NBPP rates (cases per 100,000) were estimated using U.S. Census Bureau population denominators and adjusted for the proportion of pneumonia patients tested by UAT and the number of pneumonia admissions in the catchment area.
Results
Between 2013 and 2017, 4,430 NBPP cases were identified. From 2013 to 2014, rates of NBPP declined from 153 to 90 (41% reduction, 95%CI 30%, 51%) in ≥65 year-olds; 60 to 40 (34% reduction, 95%CI 22%, 45%) in 50-64 year-olds; and 15 to 10 (36% reduction, 95%CI 25%, 47%) in 18-49 year-olds. From 2014 to 2017, rates of NBPP increased in all ages but remained below 2013 rates (Figure).

Conclusions
Reductions in NBPP among adults were primarily due to indirect effects of PCV13 use in children, with no additional declines following PCV13 introduction for adults aged ≥65 years.
CHANGES IN GENOMIC EPIDEMIOLOGY AND ANTIMICROBIAL RESISTANCE OF INVASIVE SEROTYPE 19A PNEUMOCOCCI IN THE ELDERLY AND YOUNG CHILDREN IN FINLAND AFTER PCV10 INTRODUCTION (ID 365)
Abstract
Background
Following introduction of 10-valent pneumococcal conjugate vaccine (PCV10) into Finnish infant vaccination program in 2010, serotype 19A invasive pneumococcal disease (IPD) increased particularly among older adults. We studied changes in genomic epidemiology and antimicrobial resistance of 19A-IPD isolates among elderly and children.
Methods
All 19A-IPD isolates from adults ≥65 years and children <5 years sent routinely to the national reference laboratory before (2007–2008) and after (2013–2015) PCV10-introduction were analyzed. Antimicrobial susceptibility was determined by agar dilution method using EUCAST breakpoints. Multilocus sequence typing profiles were derived from whole-genome sequencing data using Ridom SeqSphere+.
Results

Before PCV10-introduction, the most prevalent 19A clones were ST482 (antimicrobial susceptible) and ST193 (resistant to erythromycin, clindamycin, and tetracycline). During PCV10-period, antimicrobial susceptible ST199 and ST994 predominated among elderly, whereas ST994 and ST671-SLV (non-susceptible to penicillin and resistant to erythromycin) dominated among children. No increase in multidrug-resistant ST320 or ST230 was detected.
Conclusions
The genomic structure of 19A IPD isolates of elderly patients changed after infant PCV10-introduction. Although the changes resembled those observed in children, the most prevalent clones were not entirely the same in both age groups. Besides infant vaccination, also other factors like antimicrobial resistance or comorbidities/other predisposing factors in older adults may explain these differences.
IMPACT OF PNEUMOCOCCAL CONJUGATE VACCINE (PCV-10) ON RADIOLOGICAL PNEUMONIA AT A TERTIARY CARE CENTRE IN NEPAL (ID 514)
- Puja Amatya, Nepal
- Michael J. Carter, United Kingdom
- Matthew Smedley, United Kingdom
- Meeru Gurung, Nepal
- Sarah Kelly, United Kingdom
- Merryn Voysey, United Kingdom
- David Murdoch, New Zealand
- Ganesh Shah, Nepal
- Stephen Thorson, Nepal
- Shrijana Shrestha, Nepal
- Bibek Khadka, Nepal
- Animesh K. Basnet, Nepal
- Sunaina Gurung, Nepal
- Maria D. Knoll, United States of America
- Dominic Kelly, United Kingdom
- Andrew J. Pollard, United Kingdom
- Kate M. Park, United Kingdom
Abstract
Background
Routine immunization with 10-valent pneumococcal conjugate vaccine (PCV10) was introduced in Kathmandu in 2015 with doses administered at 6 weeks, 10 weeks and 9 months of age. We assessed the impact of PCV10 on the prevalence of radiographic changes in children aged 2 months to 14 years with a clinical diagnosis of pneumonia admitted to Patan Hospital, Kathmandu.
Methods
Digitalized chest radiographs were interpreted using standardized WHO criteria as primary endpoint pneumonia (PEP), other infiltrate or normal, by two specific readers. A third reader arbitrated upon all discordant results.
Results
From March 2014 to December 2018, 1755 children were enrolled, of whom 1692 (96%) had interpretable radiographs. The proportion of children with PEP decreased annually from 84/189 (44%) in 2014 to 105/414 (25%) in 2018 (p<0.001). PEP was associated with age, occurring in 247/1090 (22%) children <2 years of age, in comparison with 120/175 (69%) children ≥5 years of age (p<0.001), and carriage of PCV10 serotypes, occurring in 95/188 (51%) children with PCV10 carriage in comparison with 459/1504 (31%) children with non-PCV10 serotypes or no carriage (p<0.001).
Conclusions
The prevalence of PEP in children hospitalized with pneumonia decreased from 2014 to 2018 in association with the implementation of PCV10 immunization in Kathmandu.
THE IMPACT OF PNEUMOCOCCAL CONJUGATE VACCINE INTRODUCTION IN NEPAL: A SIX-YEAR PAEDIATRIC SURVEILLANCE STUDY (ID 516)
- Shrijana Shrestha, Nepal
- Meeru Gurung, Nepal
- Stephen Thorson, Nepal
- Bhishma Pokhrel, Nepal
- Bibek Khadka, Nepal
- Pratistha Maskey, Nepal
- Puja Amatya, Nepal
- Madhav C. Gautam, Nepal
- Michael J. Carter, United Kingdom
- Rama Kandasamy, Australia
- Brian Wahl, United States of America
- Sarah Kelly, United Kingdom
- Krishna G. Prajapati, Nepal
- Sonu Shrestha, United Kingdom
- Maria Deloria Knoll, United States of America
- Jason Hinds, United Kingdom
- Ganesh Shah, Nepal
- Dominic Kelly, United Kingdom
- David Murdoch, New Zealand
- Merryn Voysey, United Kingdom
- Andrew J. Pollard, United Kingdom
Abstract
Background
S. pneumoniae is a major cause of bacterial pneumonia and an important cause of invasive bacterial disease (IBD) in children under-five years of age in Nepal. Pneumococcal conjugate vaccine, PCV10, was introduced in 2015 with a 2+1 schedule.
Methods
We assessed the programmatic impact of PCV10 introduction using surveillance for nasopharyngeal (NP) colonisation, pneumonia and IBD. NP swabs from pneumonia inpatients and from healthy children, blood cultures from inpatients with suspected IBD, and chest x-rays from inpatient pneumonia cases were obtained over a 6-year period (2014-2019).
Results
The proportion of pneumonia cases with radiographic endpoint-consolidation (likely bacterial) was 34% lower (95%CI 19-46%) in 2018 compared with the pre-vaccine period (2014-2015). Vaccine serotype (VT) carriage in children under 2-years of age with pneumonia in 2019 was 78% lower (95%CI 30-93%) than in the pre-vaccine period.
Among healthy 6-23 month old children (urban and rural cohorts), VT-carriage declined 74% (95%CI 43-82%) by 2019. An increase in PCV13-additional-serotype carriage was seen in 2018 among rural-children (prevalence-ratio 1.65, 95%CI 1.17-2.32), but not urban-children.
Serotype 1 remains the dominant serotype detected in cases of invasive pneumococcal disease.
Conclusions
A decrease in prevalence of endpoint-consolidation-pneumonia and a decrease in vaccine-serotype circulation have been observed post PCV introduction in Nepal.
IMPACT OF PCV10 INTRODUCTION ON NASOPHARYNGEAL CARRIAGE OF STREPTOCOCCUS PNEUMONIAE IN HEALTHY CHILDREN IN RURAL AND URBAN NEPAL (ID 531)
- Madhav Chandra Gautam, Nepal
- Sonu Shrestha, United Kingdom
- Sanjeev M. Bijukchhe, Nepal
- Meeru Gurung, Nepal
- Bhishma Pokhrel, Nepal
- Merryn Voysey, United Kingdom
- Peter J. O'Reilly, United Kingdom
- Sarah Kelly, United Kingdom
- Ganesh Shah, Nepal
- Laxmi Lama, Nepal
- Pratistha Maskey, Nepal
- Stephen Thorson, Nepal
- David Murdoch, New Zealand
- Maria Deloria Knoll, United States of America
- Dominic Kelly, United Kingdom
- Shrijana Shrestha, Nepal
- Andrew J. Pollard, United Kingdom
Abstract
Background
The ten-valent pneumococcal conjugate vaccine (PCV10) was introduced in Nepal in 2015. We compared the nasopharyngeal carriage of PCV10 and non-PCV10 serotypes of pneumococcus between pre-vaccine (2015) and post-vaccine (2017-2018) years in two different regions of Nepal.
Methods
Nasopharyngeal samples obtained in healthy Nepalese children aged 6-59 months in urban (Patan, Kathmandu) and 6-23 months in rural (Okhaldhunga) settings were transported in STGG (Skim Milk-Tryptone-Glucose-Glycerol) media, cultured for pneumococcus and serotyped by the Quellung method.
Results
The carriage prevalence decreased for all PCV10-type serotypes except 7F in both the settings. PCV10-type prevalence decreased from 29.7% in rural and 17.2% in urban children pre-vaccine to 9.0% and 8.6% post-vaccine, respectively. Pre-vaccine, the most frequently found serotypes in both settings were 19F, 6B, 14. Post-vaccine, the non-PCV10 serotypes were more common; serotypes 34, 6C, 19A and 15B were most common in rural and 6A, 34, 11A, 6C and 15B in urban settings.
Conclusions
Since the introduction of PCV10, carriage prevalence of PCV10 serotypes have reduced and non-PCV10 serotypes have increased in both settings raising the possibility of replacement disease. Continued monitoring of changes in PCV10-serotypes and non-PCV10 serotypes, especially those covered by PCV13, is important to assess vaccine impact.
IMPACT OF 10-VALENT PNEUMOCOCCAL CONJUGATE VACCINE INTRODUCTION ON INVASIVE PNEUMOCOCCAL DISEASE (IPD) IN NEPALESE CHILDREN (ID 563)
- Meeru Gurung, Nepal
- Merryn Voysey, United Kingdom
- Stephen Thorson, Nepal
- Sanjeev M. Bijukchhe, Nepal
- Peter J. O'Reilly, United Kingdom
- Michael J. Carter, United Kingdom
- Bibek Khadka, Nepal
- Animesh Khulal, Nepal
- Sunaina Gurung, Nepal
- Bhishma Pokhrel, Nepal
- Ganesh Shah, Nepal
- Imran Ansari, Nepal
- Sarah Kelly, United Kingdom
- Dominic Kelly, United Kingdom
- David Murdoch, New Zealand
- Andrew J. Pollard, United Kingdom
- Shrijana Shrestha, Nepal
Abstract
Background
We assessed the distribution of pneumococcal serotypes in children with microbiologically-confirmed invasive pneumococcal disease (IPD) before (2014-2015) and after (2016-2019) PCV10 introduction in Nepal in 2015.
Methods
Children (aged 2 months to <14 years) admitted to Patan Hospital, Nepal with pneumococcus detected in blood, CSF or pleural fluid were included. Serotyping was by Quellung method.
Results
Pre-vaccine, 6/22 (27.3%) IPD cases were age <2 years; post-vaccine, 5/36 (13.9%) were <2 years. Ratio of vaccine-type to non-vaccine-type IPD among <2y olds was 5:1 pre-vaccine and 2:3 post-vaccine; among >=2y olds, the ratio was 13:1 pre-vaccine and 7:1 post-vaccine. Most (32/41, 78%) vaccine-type IPD was serotype 1: 3/7 among <2 year olds (n=1 post-vaccine); 29/34 among >=2 year olds (n=17/19 post-vaccine were >4 years old). Among 44 IPD cases detected from blood, 36 (82%) were vaccine-type (n=29 were ST1), and 7 were non-vaccine-type (6C, 10A (n=2), 19A, 24F, 38, 41). Of 13 detected from CSF (1 culture, 3 PCR and 9 Binax-only), 5 were serotyped (1, 14, 6B, 6A/B, 7F) .The 3 pleural fluid cases were serotypes 1 (n=2) and 19A.
Conclusions
Post-PCV10 introduction, IPD among <2 year olds fell; although a high proportion of ST1 IPD remains, most were >4 years old.
HIGH RATES OF MULTIPLE NASOPHARYNGEAL PNEUMOCOCCAL CARRIAGE IN CHILDREN WITH PNEUMONIA IN PAPUA NEW GUINEA FOLLOWING PNEUMOCOCCAL CONJUGATE VACCINE INTRODUCTION (ID 731)
- Rebecca Ford, Papua New Guinea
- Eileen M. Dunne, United States of America
- Jocelyn Chan,
- Lapule Yuasi, Papua New Guinea
- Mition J. Yoannes, Papua New Guinea
- Casey L. Pell, Australia
- Ahmed Alamrousi, Australia
- Jason Hinds, United Kingdom
- Joycelyn J. Sapura, Papua New Guinea
- Birunu Nivio, Papua New Guinea
- Zeena Akunaii, Papua New Guinea
- Kim E. Mulholland, Australia
- Deborah Lehmann, Australia
- William Pomat, Papua New Guinea
- Christopher C. Blyth, Australia
- Catherine Satzke, Australia
- Fiona M. Russell, Australia
Abstract
Background
Pneumococcal carriage rates in Papua New Guinean (PNG) children are among the highest globally. One aim of the multi-site PneuCAPTIVE study is to determine the impact of PCV13 (introduced in 2014) on nasopharyngeal carriage in PNG.
Methods
Nasopharyngeal (NP) swabs and blood were collected from children aged <5 years with moderate or severe pneumonia, and/or suspected meningitis at Eastern Highlands Provincial Hospital or outpatient clinics in Goroka (2016-2018). Pneumococci were identified and quantified by lytA qPCR, and serotyped by microarray. IPD was identified by standard blood culture.
Results
PCV13 coverage was 62%. 1043 were enrolled: 90% had pneumococcal carriage, with median density of 6.59 log10 genome equivalents (GE)/ml (IQR 6.00-7.11). Serotype data were available on 914 cases: 37% were PCV13-types; and 55% had multiple pneumococcal-type carriage. 74 different serotypes and genetic lineages of acapsular pneumococci were identified, the most common being acapsular lineage NT2>19A>15B/C>16F>14. PCV13-type carriage was 28% in vaccinated children vs 46% in unvaccinated children. IPD was confirmed in 7 cases (vaccinated – serotype 1; unvaccinated – serotypes 2, 6B, 15F, 19A, 23A, 29): 4/7 carried the homologous serotype.
Conclusions
There is some evidence of PCV13 being effective against PCV13-types but the high diversity of serotypes in PNG warrants extended valency vaccines.
EPIDEMIOLOGY OF INVASIVE PNEUMOCOCCAL DISEASE (IPD) FOLLOWING 18 YEARS OF PNEUMOCOCCAL CONJUGATE VACCINE (PCV) USE IN THE UNITED STATES (ID 849)
- Ryan Gierke, United States of America
- Monica Farley, United States of America
- William Schaffner, United States of America
- Ann Thomas, United States of America
- Arthur Reingold, United States of America
- Lee Harrison, United States of America
- Corinne Holtzman, United States of America
- Kari Burzlaff, United States of America
- Susan Petit, United States of America
- Rachel Herlihy, United States of America
- Salina Torres, United States of America
- Bernard Beall, United States of America
- Tamara Pilishvili, United States of America
Abstract
Background
PCVs have been recommended for U.S. children since 2000 and for adults aged ≥65 years since August 2014. We evaluated PCV impact on IPD.
Methods
IPD cases (isolation of pneumococcus from sterile sites) were identified through CDC’s Active Bacterial Core surveillance during 1998-2018. Isolates were serotyped by Quellung or whole genome sequencing and classified as PCV13-type and non-vaccine-type (NVT). Incidence rates (cases/100,000) were calculated using U.S. Census Bureau population denominators.
Results
During 1998-2018, overall and PCV13-type IPD rates declined significantly among children and adults aged ≥65 years (Figures); serotypes 3, 19A, and 19F caused most of the remaining PCV13-type IPD. NVT IPD rates did not change. The most common NVTs in 2018 were 22F (10% of all IPD), 9N (7%) and 15A (5%). Among children, the proportion of cases with meningitis increased from 5% to 14%(p<0.01), and the proportion with pneumonia/empyema increased from 17% to 31%(p<0.01). Among adults, the proportion of cases with meningitis did not change (3%), while the proportion with pneumonia/empyema increased from 72% to 76%(p=0.01).

Conclusions
Overall IPD incidence among children and adults decreased following PCV introduction for children, driven primarily by reductions in PCV-type IPD. Increases in NVT IPD were minimal compared with PCV benefits.
TWENTY YEARS OF PAEDIATRIC NASOPHARYNGEAL PNEUMOCOCCAL CARRIAGE IN REMOTE NORTHERN AUSTRALIA; SEROTYPES AND ANTIMICROBIAL RESISTANCE IN CHANGING PCV ERAS (ID 933)
Abstract
Background
Invasive pneumococcal disease and otitis media due to pneumococci disproportionately affect remote dwelling First Nations Australian children, driven by nasopharyngeal colonisation by respiratory pathogens in the first weeks of life. Microbiological samples have been collected from surveillance studies and clinical trials since 1996. We aimed to explore trends in nasopharyngeal (NP) pneumococcal carriage over time, and factors associated with serotype distribution and resistance.
Methods
This study collated NP microbiological data from the non-interventional arms of trials and cross-sectional studies. Carriage and serotype-specific rates by age category in the differing conjugate vaccine eras will be described. Hierarchical logistic regression will be used to account for confounders (age, clustering within individuals and communities).
Results
Interim findings from over 9,000 NP swabs from children with a median age of 19 months (range 0-18 years) suggest expected reductions in vaccine type pneumococci, with no significant change in overall carriage rates, replacement by non-vaccine serotypes with persistent low-level carriage of vaccine types. 16F was the most commonly isolated serotype, with 50% non-susceptible to penicillin.
Conclusions
Understanding the impact of PCVs on serotype dynamics and antibiotic non-susceptibility of S.pneumoniae in this remote-dwelling population will strengthen evidence for vaccine policy and ongoing antimicrobial stewardship in the region.
MONITORING PCV13 IMPACT USING NASOPHARYNGEAL CARRIAGE SURVEILLANCE AMONG CHILDREN WITH PNEUMONIA IN MONGOLIA (ID 965)
- Purevsuren Batsaikhan, Mongolia
- Jocelyn Chan,
- Tuya Mungun, Mongolia
- Eileen M. Dunne, United States of America
- Sophie La Vincente, Australia
- Dorj Narangerel, Mongolia
- Monica L. Nation, Australia
- J Hinds,
- Cattram D. Nguyen, Australia
- Mukhchuluun Ulziibayar, Mongolia
- Catherine Satzke, Australia
- Kim E. Mulholland, Australia
- Claire Von Mollendorf, Australia
- Fiona M. Russell, Australia
Abstract
Background
In 2015, Mongolia was among the earliest countries in Asia to introduce PCV. To monitor the impact of PCV13 introduction, we commenced nasopharyngeal carriage surveillance among children with pneumonia 6 months prior to vaccine introduction.
Methods
We recruited children 2-59 months of age presenting with pneumonia to district hospitals and the national Maternal and Child Health hospital in two districts in Ulaanbaatar. Clinical and demographic data, vaccination status and nasopharyngeal swabs were collected. A random sample of swabs were selected for testing each month. Samples were examined by lytA qPCR, with positives serotyped by microarray.
Results
We recruited 4980 children and tested 983 children from November 2015 to April 2018. The median age was 1.27 and 25.81% of cases were vaccinated in the first and second year following PCV13 introduction, respectively. 474 and 48.22% had received antibiotics in the 48 hours before admission.
Conclusions
Following PCV13 introduction in Mongolia, the prevalence of pneumococcal carriage remained stable while the prevalence of PCV13-type carriage decreased among children with pneumonia. Reductions in PCV13 carriage likely correspond to reductions in disease due to PCV13 types, since carriage is a precursor for disease.
LONG-TERM TRENDS IN NASOPHARYNGEAL CARRIAGE OF VACCINE-TYPE PNEUMOCOCCI: FINDINGS FROM A MATURE 10-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV10) PROGRAM IN KENYA (ID 966)
Abstract
Background
PCV10 was introduced into Kenya’s immunization program in 2011, with catchup in children aged 1-4 years. We evaluated the long-term effect of PCV10 on nasopharyngeal carriage of Streptococcus pneumoniae serotypes included in PCV10.
Methods
Population-based annual cross-sectional nasopharyngeal carriage surveys were conducted in randomly selected individuals between 2009 and 2019 (N= ~1000 in 2019; N= ~500 in all others) in Kilifi, Kenya. Pneumococcal identification was performed per WHO standards. Annual vaccine-type carriage prevalence was modelled using log-binomial regression with a curved function for year and adjustment for age-specific sampling probability.
Results
Compared to 2010, carriage of PCV10-type pneumococci declined significantly through 2019 in children aged <5 years to 6.1% (adjusted prevalence ratio 0.18, 95%CI 0.11-0.30) but not in children aged 5-14 years (prevalence= 7.1%; 0.71, 0.38-1.34) nor adults ≥15 years (prevalence= 1.0%; 0.49, 0.17-1.35). PCV10-type carriage was significantly lower in 2017 compared to 2010 for all age groups and did not differ from carriage prevalence in 2019 (figure).

Conclusions
PCV10-type carriage prevalence appears to be approaching equilibrium, yet residual carriage persists. Virtual elimination of PCV10-type carriage (≤1% in children <5 years; ≤3% in children 5-9 years) – a prerequisite for introduction of reduced dose schedules – is unlikely without implementation of additional strategies.
SEROTYPE DISTRIBUTION IN INVASIVE PNEUMOCOCCAL DISEASE BEFORE AND AFTER INTRODUCTION OF PCV10 IN CHILDHOOD VACCINATION IN ICELAND (ID 987)
Abstract
Background
Iceland introduced PCV10 in 2011. The aim of this study was to monitor pneumococcal serotype distribution and antimicrobial resistance in invasive pneumococcal disease (IPD) eight years before (2004-2011; PreVac) and after vaccination (2012-2019; PostVac).
Methods
All IPD samples (blood, cerebrospinal fluid and joint fluid) were recorded at the reference laboratory, Landspitali University Hospital. All isolates were serotyped with Immulex Pool Antisera and/or multiplex PCR.
Results
In total, 564 IPD cases were detected, thereof 75 cases PreVac and 15 PostVac from children (<18y) and PCV10 serotypes were 82.7% and 13.3%, respectively (p<0.001). In children <2 years, 55 IPD cases were recorded PreVac and six PostVac (three of serotype 19A and one of each 14, 22F and 23A).
In adults, 270 cases were detected PreVac and 204 PostVac, thereof PCV10 serotypes 63.7% and 23.0%, respectively (p<0.001). The most common non-PCV10 serotypes in adults PostVac were 19A (12.3%), 22F (11.3%), 9N (7.4%) and 3 (6.4%).
Penicillin non-susceptible pneumococci (PNSP) Pre- and PostVac were 29/345 (8.4%) and 30/219 (13.7%), respectively (p=0.036). The most common PNSPs PostVac were 19A (23.3%), 15A (16.7%), 6C (13.3%) and 35B (10.0%).
Conclusions
PCV10 impact was greatest among children <2 years. The increasing prevalence of penicillin non-susceptible pneumococci of non-PCV10 serotypes is concerning.
GLOBAL PREVALENCE OF ANTIMICROBIAL RESISTANCE IN PEDIATRIC STREPTOCOCCUS PNEUMONIAE ISOLATES BEFORE AND AFTER PNEUMOCOCCAL CONJUGATE VACCINE IMPLEMENTATION: A SYSTEMATIC REVIEW AND META-ANALYSIS (ID 1005)
Abstract
Background
Pneumococcal diseases are leading causes of morbidity and mortality among children globally, which may be worsened by antimicrobial resistance (AMR). Pneumococcal conjugate vaccines (PCVs) prevent antibiotic use associated with treatment of acute respiratory infections. We assessed regional changes in AMR prevalence in pediatric pneumococcal isolates globally before and after PCV implementation.
Methods
We conducted a systematic review and meta-analysis assessing the prevalence of AMR in pneumococcal isolates among children ages <18y. Population-based studies of invasive pneumococcal disease or asymptomatic nasopharyngeal colonization were eligible. We assessed changes in prevalence of isolates with reduced susceptibility or resistance to major antibiotic classes via meta-regression.
Results
Of 3,205 studies identified by our search, we extracted data from 393 eligible studies that reported on AMR prevalence across 648,317 pediatric isolates. Higher prevalence of penicillin and macrolide non-susceptibility was evident in colonizing than invasive isolates within the same regions. A significant reduction in macrolide nonsusceptibility among colonizing isolates was observed in high-income settings ≥3y after PCV implementation. However, there was inconsistent evidence of post-vaccination changes in prevalence of non-susceptibility to these drugs elsewhere, in part owing to increases in nonsusceptibility among nonvaccine-serotype pneumococci. 

Conclusions
Despite reducing disease burden, PCVs have not fully offset AMR selection in pneumococci.
PNEUMOCOCCAL SEROTYPES IN INFANTS LESS THAN 90 DAYS OLD BEFORE AND AFTER INTRODUCTION OF PNEUMOCOCCAL CONJUGATE VACCINE (PCV) IN BLANTYRE, MALAWI (ID 1071)
Abstract
Background
Malawi introduced PCV13 into its Expanded Program on Immunization in November 2011. Our aim was to describe the change in serotype distribution causing invasive pneumococcal disease (IPD) in neonates and young infants in Blantyre, Malawi.
Methods
We conducted a retrospective study of IPD in infants aged <90 days admitted to Queen Elizabeth Central Hospital (QECH) in Blantyre, Malawi between 2005 and 2018. IPD was defined as culture-confirmed Streptococcus pneumoniae from blood or cerebrospinal fluid, collected when clinically indicated. Serotype was determined by Latex agglutination.
Results
We identified and serotyped samples of 130 cases of IPD. Results show that overall, serotypes 5 (19%), 1 (11%), 7F (5%), 6A/B (3%), and 23F (3%) were most common in this population over the time period studied. Non-vaccine serotype (NVT) accounted for 19% of cases and 40 samples were not typeable. Serotypes 5 and 1 were the predominant cause of IPD before as well as after PCV13 introduction.
Conclusions
Overall IPD cases have been on the decline in this population. Vaccine-type serotypes were the main cause of IPD in neonates and young infants, both before and after PCV13 introduction. Vaccine strategies should be considered to optimise the potential for reducing VT-IPD in this setting among this vulnerable population.
RISK FACTORS FOR SEVERE PNEUMONIA IN CHILDREN 2-59 MONTHS OF AGE IN THE PNEUMOCOCCAL CONJUGATE VACCINE ERA IN ULAANBAATAR, MONGOLIA, 2015-2019 (ID 1087)
Abstract
Background
Respiratory infection is the most common cause of childhood hospitalisation in Mongolia. Mongolia initiated a staged introduction of the 13-valent pneumococcal conjugate vaccine (PCV13) into the routine immunisation programme in Ulaanbaatar from 2016. We aimed to describe the risk factors associated with severe pneumonia in children aged 2-59 months in the period surrounding PCV13 introduction.
Methods
Hospitalised children aged 2-59 months who met a predefined case definition for pneumonia were consented and enrolled at five hospitals between April 2015 and November 2019. Severe pneumonia was diagnosed in children with cough/difficulty breathing and tachypnoea plus lower chest indrawing or general danger signs or hypoxia (oxygen saturation < 90%).
Results
Over the analysis period total of 14,923 children were enrolled in the surveillance programme. Of these children 56% (8,283) had severe pneumonia and 70% (10,502) were <2 years of age. Factors associated with severe pneumonia included district of residence, child’s age (decreasing odds with increasing age), pre-existing asthma, use of household coal/wood, high C-reactive protein (>40) and winter season.
Conclusions
We identified a number of risk factors associated with severe pneumonia in the surveillance programme in Mongolia. It is important that high risk children are vaccinated and risk factors are monitored in the ongoing programme.
FACTORS ASSOCIATED WITH VACCINE TYPE PNEUMOCOCCAL CARRIAGE IN CHILDREN UNDER 2 YEARS OF AGE IN A RURAL POPULATION IN PAKISTAN (ID 1092)
Abstract
Background
Pneumococcal carriage is a prerequisite for disease with children being the main reservoir and transmitters. Here, we look at factors associated with Vaccine type (VT) carriage in children under 2 years of age in a rural population in Pakistan.
Methods
Children were enrolled and nasopharyngeal swabs collected using standard WHO guidelines. Serotyping was done using CDC standardized Multiplex PCR. The serotypes were classified as vaccine type (VT) and non-vaccine type (NVT) based on their inclusion in the ten valent vaccine (PCV10).
Results
From 2014-2018, 3140 children were enrolled. Factors negatively associated with VT carriage were: primary education of 1 to 5 years (aOR 0·7, 95%CI 0·5-0·9), history of difficulty in breathing (aOR 0·7, 95%CI 0·6-0·9), exposure to smoke (aOR 0·8, 95% CI 0·6-0·9), child fully immunized (aOR 0·7, 95%CI 0·5-0·9) and being enrolled in 3rd (aOR 0·6, 95%CI 0·4-0·8) and 4th year of study (aOR 0·6, 95%CI 0·5-0·9) whereas history of runny nose was positively associated (aOR 1·6, 95% CI 1·2-1·9).


Conclusions
Various socio-demographic and clinical factors were associated with VT carriage. A child having received all three doses of PCV10 significantly reduced the odds of carrying a VT serotype.
SERO-REPLACEMENT WITH NON-VACCINE SEROTYPES POST INTRODUCTION OF PCV10 IN NASOPHARYNX OF CHILDREN IN A RURAL COMMUNITY IN PAKISTAN (ID 1104)
Abstract
Background
Ten-valent pneumococcal vaccine (PCV10) was introduced in Pakistan’s immunization program in 2012. It is important to monitor changing sero- epidemiology post introduction for relevance of existing vaccine formulation.
Methods
From 2014 to 2018, children under the age of 2 years were enrolled from a rural district in Pakistan. Nasopharyngeal swabs were collected and serotyping was done using Multiplex PCR.
Results
Of the 3140 children enrolled, pneumococcal isolates were detected in 2370(75%). VT carriage decreased from 16·1% to 9·6% (p-value< 0·001) over 4 years. There was a significant decline in VT serotypes 6B, 9V/9A and 19F only. The carriage of serotype 19A significantly increased from 4.0% to 6.8% (p-value< 0·001). This increase was more pronounced in vaccinated group. There was no significant change in the ten most common NVT serotypes except for 10A, which increased over time. There was no decline noted in carriage rate for serotype 6A.
Conclusions
We observed an ongoing shift in the pneumococcal carriage of serotypes in children in the rural community. Since the pneumococcal serotypes associated with carriage and Invasive disease are constantly changing, monitoring is necessary to assess the impact of the vaccine and to develop better vaccination strategies.

LONG-TERM IMPACT OF THE TEN-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV10) ON OUTPATIENT ANTIMICROBIAL PRESCRIPTIONS IN THE NATIONAL VACCINATION PROGRAMME (NVP) IN FINLAND (ID 230)
Abstract
Background
Respiratory infections are the most common cause of antimicrobial prescriptions in children. We have earlier reported 18% reduction in antimicrobial use. Now, we evaluated the long-term impact of PCV10 introduction into the Finnish NVP on antimicrobial use in vaccine-eligible children.
Methods
PCV10-NVP began 09/2010 (2+1 schedule) with high uptake at 92-95%. The target cohort eligible for NVP (children born 06/2010-09/2016) was compared with calendar-time and age-matched (3-78 months) reference cohort before NVP introduction (Figure) 3-6 years after PCV introduction. Purchase data were collected from the Social Insurance Institution of Finland reimbursement register. We assessed the trends in outpatient purchases of antimicrobials recommended for treatment of acute otitis media in the national guidelines (amoxicillin with/without enzyme inhibitor, cefuroxime, cefaclor, clarithromycin, azithromycin) but complete data on penicillin, and sulfadiazine/trimethoprim were not available.

Results
Rates of antimicrobial purchases were 0.93 and 0.66 per person-year in the reference and target cohorts, respectively. The relative and absolute rate reductions were 29.1% (95%CI 28.8-29.3) and 0.27 per person-year, respectively.
Conclusions
Continuous reduction in antimicrobial use observed since PCV introduction is compatible with the development of indirect effects. Although the decline started soon after PCV introduction, other reasons may also have contributed to the decline.
THE LOW PNEUMOCOCCAL CARRIAGE PREVALENCE AMONG COMMUNITY-DWELLING OLDER PEOPLE IN JAPAN (ID 262)
Abstract
Background
Pneumococcal colonization is considered an initial step of the development pneumococcal diseases. However, the carriage prevalence of pneumococcus among community-dwelling older adults is not fully understood. The objectives of this study were to investigate the carriage prevalence of pneumococcus in the upper respiratory tract and to identify the risk factors of the carriage among community-dwelling adults aged ≥65 years in Japan.
Methods
A cross-sectional study of community-dwelling adults aged ≥65 years was conducted from February 2018 to December 2018 in Nagasaki city, Japan. We collected demographic and clinical data and nasopharyngeal, oropharyngeal and saliva samples from each participant. The specimens were tested by culture and molecular methods.
Results
A total of 504 participants were enrolled during the study period. None were positive for pneumococcus by culture, and 22 were positive by PCR. Therefore, the overall pneumococcal carriage prevalence was 4.4% (95% CI: 2.8-6.5%). No demographic characteristics were significantly associated with carriage prevalence, including age (4.7% among participants aged 65-74 years and 4.1% among those 75 years and older). Each serotype of the pneumococcal-positive samples was identified, and PCV13-covered serotypes accounted for 18.2%.
Conclusions
Our study showed a low pneumococcal carriage prevalence among community-dwelling older people in Japan.
CHANGES IN SEROTYPE DISTRIBUTION OF INVASIVE PNEUMOCOCCAL DISEASE IN COLOMBIA AFTER MASS VACCINATION WITH PCV10 (ID 278)
- German Camacho Moreno, Colombia
- Aura L. Leal Castro, Colombia
- Jaime A. Patiño Niño, Colombia
- Vivian Marcela Moreno Mejia, Colombia
- Ivan F. Gutierrez Tobar, Colombia
- Cristina Mariño, Colombia
- Sandra Beltran, Colombia
- Martha I. Alvarez-Olmos, Colombia
- Rocio Barrero Barreto, Colombia
- Juan P. Rojas, Colombia
- Fabio Espinosa, Colombia
- Catalina Arango, Colombia
- Maria A. Suarez, Colombia
- Monica Trujillo, Colombia
- Eduardo López, Colombia
- Pio López, Colombia
- Wilfrido Coronell, Colombia
- Hernando Pinzon, Colombia
- Nicolas Ramos, Colombia
- Anita Montañez, Colombia
Abstract
Background
Invasive Pneumococcal Disease(IPD) causes high morbidity and mortality in children under 5 years. Colombia implemented PCV10 in 2012, and Neumocolombia network monitors IPD in pediatric patients throughout the country.
Methods
Ambispective case series study conducted in pediatric patients with IPD admitted to 10 hospitals of Bogotá (2008-2019), and 4 hospitals of Cali, 2 of Medellín, and 1 of Cartagena (2017-2019). Preliminary data on serotype(Spn) and resistance were obtained.
Results
651 patients were included. Serotyping was obtained in 417(64%); the most frequent serotypes were 19A(26.8%), 14(15.5%), 3(10.3%); and 1(10%). IPD prevalence in serotype 14 decreased from 35.3% (41/116) in 2008-2011 to 6.4% (6/93) in 2012-2014 and to 8.9% (18/202) in 2015-2019. Serotype 1 prevalence decreased from 18.12% (21/116) in 2008-2011, to 19.3%(18/93) in 2012-2014 and to 1.4%(3/202) in 2015-2019. Spn19A increased from 4.3%(5/116) in 2008-2011 to 10.7%(10/93) in 2012-2014 and to 56%(112/202) in 2015-2019. Spn3 increased from 3.4%(4/116) in 2008-2011 to 11.8%(11/93) in 2012-2014, and to 13.8%(28/202) in 2015-2019.
Conclusions
The prevalence of serotypes 14 and 1, included in PCV10, decreased, while serotypes 19A and 3 increased. These findings are relevant for IPD epidemiology, and, according to the WHO, they should promote a change in the immunization schedule with a vaccine against these serotypes.
COMPARATIVE EFFECTIVENESS OF PCV7, PCV10, AND PCV13 - A HEAD-TO HEAD COHORT STUDY (ID 354)
Abstract
Background
‘Before-after’ studies comparing effectiveness of different pneumococcal conjugate vaccines (PCVs) are limited because they cannot control for changes in disease risk that occur over time. Since 2008 New Zealand (NZ) have transitioned through PCV7, PCV10, PCV13, and back to PCV10. To provide comparative effectiveness estimates and minimise changes in disease risk we conducted a retrospective cohort study based on populations of NZ babies who received a PCV during two transition periods where different brands of PCV were in use at the same time.
Methods
Administrative health data provided vaccination information, covariates and outcome measures (hospitalization with pneumonia or OM). Cox’s proportional hazards regression provided effectiveness estimates and ratios.
Results
Over 53,000 eligible children were vaccinated during the transition periods. In 6 years of follow up (PCV7-PCV10 transition) there were 3,425 OM hospitalisations and 1,763 all-cause pneumonia hospitalisations. PCV10 was better at preventing OM hospitalisation compared to PCV7 (HR 0.89, 95% CI 0.82-0.97). We followed babies born during the second (PCV10-PCV13) transition for 18 months, there were no significant differences in effectiveness for any of our considered outcomes; hospitalisaion with OM, all-cause pneumonia or bacterial pneumonia.
Conclusions
PCV10 and PCV13 are both equally protective against OM and pneumonia in the NZ setting.
IMPACT OF PCV13 ON THE INCIDENCE OF HOSPITALIZATONS FOR ALL-CAUSE PNEUMONIA AMONG CHILDREN AGED LESS THAN 5 YEARS IN BURKINA FASO: AN INTERRUPTED TIME-SERIES ANALYSES (ID 371)
- Lassane Kabore, Burkina Faso
- Seydou Ouattara, Burkina Faso
- François Sawadogo, Burkina Faso
- Alain Gervaix, Switzerland
- Annick Galeto-Lacour, Switzerland
- Robert Karama, Burkina Faso
- Amado T. Traore, Burkina Faso
- Bertrand Meda, Burkina Faso
- Haoua Tall, Burkina Faso
- Alima T. Essoh, Burkina Faso
- Bradford D. Gessner, United States of America
- Jennifer C. Moïsi, France
Abstract
Background
Pneumococcal disease is a major public health concern globally and particularly in Burkina Faso where PCV13 was introduced nationwide into the routine immunization schedule in 2013. We sought to evaluate vaccine impact on all-cause pneumonia hospitalizations among children < 5 years.
Methods
We retrospectively collected hospitalization data over 10 years (January 1st 2009-December 31st 2018) in 4 rural district hospitals, using medical records to extract data on relevant variables. We used interrupted time series and segmented regression to estimate the effect and impact of PCV13 on the rates of pneumonia hospitalizations. Severe acute malnutrition and unintentional injury were used as control conditions.
Results
We found a vaccine effectiveness of 34% (95% CI : 16-49, p=0·001), 24% (95% CI : 2-41, p=0·032) and 50% (95% CI : 30-64, p<0·001) against all-cause pneumonia hospitalizations among children < 5 years, < 2 years, and 2-4 years, respectively. By October 2018, PCV13 had led to an absolute reduction in the pneumonia hospitalization rate of 348 cases per 100,000 among children < 5 years. No decline was observed for control conditions.
Conclusions
Our estimates point to a substantial public health impact of PCV13 against pneumonia hospitalizations among children < 5 years in Burkina Faso.
LONG-TERM POPULATION EFFECTS OF 10-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV10) PROGRAM ON INVASIVE PNEUMOCOCCAL DISEASE (IPD) IN FINLAND (ID 459)
Abstract
Background
Limited data are available on the population impact of mature PCV10 programs. We evaluated long-term changes in IPD incidence and serotypes.
Methods
Culture-confirmed IPD cases (n=9481) were identified through national, population-based laboratory surveillance; person-years of follow-up were obtained from population registry. We compared IPD rates before (7/2004-6/2010) and after (7/2011-6/2018) PCV10 introduction.
Results
IPD incidence in children <5 years decreased from 37 to 10/100,000 person-years; no PCV10 type cases were seen in 2018. Before PCV10 introduction, overall IPD incidence was increasing (p for trend <0.05; Figure). IPD rates overall and in persons >65 years were similar before and after PCV10 (Table). Non-vaccine serotypes (NVT) increased in all age groups, particularly the elderly. Serotype 19A was most common NVT in children. In adults, serotypes 3, 19A, 22F and 6C accounted for most of the NVT rate increase.
Figure: IPD incidence by serotype group from 7/2004 to 6/2018, all ages


Conclusions
After 8 years of infant PCV10 program, IPD incidence has reached a steady state with substantial reductions in children and leveling of NVT increase in adults. Herd effects also seem to have offset the pre-vaccine increase in adult IPD incidence and similar overall disease burden remains.
EFFECT OF NATIONAL INTRODUCTION OF 10-VALENT PNEUMOCOCCAL CONJUGATE VACCINE ON INVASIVE PNEUMOCOCCAL DISEASES IN BANGLADESH: A POPULATION-BASED STUDY (ID 471)
- Abdullah H. Baqui, United States of America
- Eric D McCollum, United States of America
- Arunangshu Roy, Bangladesh
- Nabidul H. Chowdhury, Bangladesh
- Sayed J. Rizvi, Bangladesh
- Rasheda Khanam, United States of America
- Meagan Harrison, United States of America
- Salahuddin Ahmed, Bangladesh
- Nazma Begum, Bangladesh
- Maksuda Islam, Bangladesh
- Abdul Quaiyum, Bangladesh
- William Checkley, United States of America
- Mathuram Santosham, United States of America
- Samir K. Saha, Bangladesh
- Lawrence Moulton, United States of America
Abstract
Background
Bangladesh introduced 10-valent pneumococcal conjugate vaccine (PCV10) in its national immunization program in early 2015. We conducted case-control, incident trend and indirect cohort analyses to assess PCV impact on invasive pneumococcal diseases (IPD).
Methods
To assess PCV impact on IPD, we established community and facility-based surveillance in Bangladesh’s Sylhet district. Children 3-35 months of age attending study clinics were assessed for suspected IPD. Blood and CSF were collected from suspected IPD cases to isolate pneumococcus using culture and molecular tests. Community and clinic controls were matched to each IPD case. Data on immunization status and potential confounders were collected from cases and controls.
Results
During July 2015-June 2018, 94,584 children 3-35-month-old were under surveillance. Of these, 32,021 sought care from study clinics. We enrolled 44 IPD cases, 158 clinic and 173 community controls. Case-control analysis using clinic controls showed 89.6% (95% CI: -26.0 to 99.1) and using community controls showed 83.1% (95% CI:1.57 to 97.1) effectiveness in preventing vaccine type IPD. Time trend analysis estimated 80.1% (95% CI: 38.4, 93.6) effectiveness, and the indirect cohort analysis estimated 76.1% (95% CI: 17.3, 93.1) effectiveness with 3 doses of PCV.
Conclusions
PCV in our population is highly effective in preventing IPD.
EFFECTIVENESS OF INFLUENZA VACCINATION AGAINST INVASIVE PNEUMOCOCCAL DISEASE IN THE ELDERLY ≥65 YEARS ELIGIBLE FOR VACCINATION IN ENGLAND AND WALES (ID 553)
Abstract
Background
The clinical and epidemiological studies conducted during the 1918 pandemic brought new evidence, with Influenza viruses recognised to induce Pneumonia and favour bacterial co-infections and secondary bacterial infections. Therefore, Influenza vaccination may protect against Pneumococcal outcomes by reducing the risk of influenza infection.
Methods
Screening method is used to measure VE. IPD cases were extracted from surveillance database with information on serotype, specimen date, age, clinical conditions, sex, PPV23 and Influenza vaccination dates. Influenza vaccine coverage was extracted from RCGP and matched to IPD cases based on age group (65-74, 75-84, ≥ 85), risk group, PPV23 vaccination, and weeks. The study period was September 2015 to June 2016.
Results
Overall crude Flu VE against IPD was 45% (39, 50). Matching IPD with RCGP proportion vaccinated, overall adjusted VE was 27% (19, 34) in 65+, 26% (14, 38) in 65-74 and 75-84, 28% (14, 40) in 85+ years old. Stratifying by time, VE was 24% (13-33) in January – April and 24% (00-42) in May - June.
Conclusions
Since VE against Influenza was 29% overall and 56% against H1N1 in the elderly in 2015/16 in UK, a very strong association between Influenza and IPD would be needed to have a 27% VE against IPD.
LARGER BENEFIT OF 13-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV13) ON VACCINE PREVENTABLE DISEASE IN ADULTS (ID 625)
Abstract
Background
In the Netherlands randomized controlled trial (RCT) of 13-valent pneumococcal conjugate vaccine (PCV13) among persons age 65+ years, the primary outcome was chest x-ray (CXR) confirmed, vaccine-type (VT) primarily non-bacteremic Community Acquired Pneumonia (CAP), the latter determined almost entirely by serotype-specific urinary antigen detection (SSUAD). The sensitivity and specificity of SSUAD and CXR for identifying primarily non-bacteremic pneumonia preventable by PCV13 is unknown.
Methods
In the Netherlands RCT, we compared vaccine-preventable-disease-incidence (VPDI) for all episodes of clinically defined CAP to that for CXR-confirmed VT-CAP. VPDI was calculated as the incidence rate difference between controls and vaccinated persons.
Results
VPDI per 100,000 person-years of follow-up for all episodes of clinical CAP vs. VT-CAP were: total population, 72.2/25.1 (2.9-fold); at-risk condition for pneumococcus other than immunosuppression, 121.4/34.3 (3.5-fold); and not at-risk, 35.5/17.6 (2.0-fold) (Table).

Conclusions
PCV13 prevented 2.0 to 3.5 more episodes of clinical CAP than CXR-confirmed VT-CAP, reflecting that SSUAD was designed for the regulatory outcome of vaccine efficacy, which relies on test specificity, and that CXR may have imperfect sensitivity for CAP in elderly persons. Assessing PCV13’s true public health value will require use of rate reductions or adjustment factors to account for clinically defined CAP.
PREVALENCE OF VACCINE-TYPE PNEUMOCOCCAL CARRIAGE FIVE YEARS AFTER 13-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV13) INTRODUCTION IN GHANA (ID 663)
- Abass Abdul-Karim, Ghana
- Katherine Fay, United States of America
- Adam Zakariah, Ghana
- Fabiana Pimenta, United States of America
- John Abenyeri, Ghana
- Winfred Ofosu, Ghana
- Franklin Asiedu-Bekoe, Ghana
- David Opare, Ghana
- Badu Sarkodie, Ghana
- M Wardle, United States of America
- J Waldorf, United States of America
- Chastity Walker, Ghana
- Sopio Chochua, United States of America
- C. G. Whitney, United States of America
- Benard Beall, United States of America
- M.D Carvalho, United States of America
- Fernanda C. Lessa, United States of America
Abstract
Background
Outbreaks of vaccine-type (VT) pneumococcal meningitis, especially serotype 1 (ST1), continue in Ghana despite PCV13 introduction in 2012 (6, 10, and 14-week schedule) and coverage >85%. We assessed VT-pneumococcal carriage prevalence, focusing on ST1, during the 2018 meningitis season
Methods
We conducted a cross-sectional nasopharyngeal (NP) carriage survey from February-July in three northern Ghana “meningitis belt” regions. Individuals aged 6 weeks-35 years with respiratory symptoms or clinical signs of meningitis were recruited from emergency departments across 6 hospitals. Pneumococci isolated from NP swabs were serotyped by Quellung; ST1 isolates were sequenced.
Results
Among 934 participants, 671 (71.8%) carried pneumococci. Overall and VT-pneumococcal carriage were 76.3% (464/608) and 21.5% (131/608) in <5 years, 67.4% (116/172) and 26.7% (46/172) in 5-14 years, and 59.1% (91/154) and 19.5% (30/154) in 15-35 years, respectively. ST1 colonization was lower in participants <5 versus ≥ 5 years-old (0.3% [2/608] vs. 3.1% [10/326]; P=0.001). All ST1 isolates were sequence type 217/303, but not closely related. Serotypes 3, 19F, 23F, 6A and 14 were the most prevalent PCV13-types.
Conclusions
VT-pneumococcal carriage remains common in Ghana, with ST1 colonization more common among those too old to have received PCV13. Vaccination strategies to decrease VT-pneumococcal transmission are needed.
EPIDEMIOLOGY OF SEROTYPES OF S. PNEUMONIAE IN PATIENTS OLDER 18 YEARS IN RUSSIA (ID 687)
Abstract
Background
Aim: To determine the spectrum of serotypes of S. pneumoniae in 18+ patients with non-invasive and invasive forms of pneumococcal infection.
Methods
S. pneumoniae strains were collected and identified by the participating laboratory. Clinical isolates were stored and sent to the reference laboratory of the Institute of Antimicrobial Chemotherapy (Smolensk, Russia). All strains of S. pneumoniae were re-identified. Serotyping of S. pneumoniae was performed using Real-Time PCR.
Results
We analyzed 316 strains of S. pneumoniae – 20 strains from middle ear fluid, 262 – from respiratory samples, and 34 – from blood samples and cerebrospinal fluid.
Сommon serotypes of S. pneumoniae in patients with AOM were: 19F (30%), 3 (15%) and 14 (10%) serotypes’ coverage of PCV13 was 70.0%, and the coverage of PPV23 was 75.0%. The most common serotypes in patients with pneumonia were 19F (15.6%), 6AB (11.4%), 3 (10.3%). PCV13’ coverage was 56.8%, PPV23 – 69.0%. Prevailing serotypes of S. pneumoniae in patients with IPD were 3 (23.5%), 23F and 6AB (each 8.9%). The coverage of PCV13 was 55.6%, PPV23’ coverage was 73.3%.
Conclusions
Vaccine serotypes of S. pneumoniae are dominating in 18+ patients in Russia. Nevertheless, we can see the reduction of PCV’s coverage of serotypes in patients with pneumonia and IPD (less than 60%).
IMPACT OF A SINGLE DOSE OF PCV10 OR PCV13 ON NASOPHARYNGEAL PNEUMOCOCCAL CARRIAGE IN VIETNAMESE CHILDREN DURING THE FIRST YEAR OF LIFE (ID 696)
- Hoan Thi Pham, Viet Nam
- Beth Temple, Australia
- Thi Trang Dai Vo, Viet Nam
- Thanh V. Phan, Viet Nam
- Loc Thuy Ho Nguyen, Viet Nam
- Anh H.V Nguyen,
- Belinda D. Ortika, Australia
- Kathryn Bright, Australia
- Catherine Satzke, Australia
- Nevio D. Sarmento, Timor-Leste
- Jemima Beissbarth, Australia
- Heidi Smith-Vaughan, Australia
- Thuong V. Nguyen, Viet Nam
- Kim E. Mulholland, Australia
Abstract
Background
Reduced-dose schedules of pneumococcal conjugate vaccine (PCV) could increase the accessibility and use of PCV in low and middle-income countries.
Methods
Groups within the Vietnam Pneumococcal Trial II receive PCV10 and PCV13 in a 1+1 schedule at 2 and 12 months of age, or no vaccine. Nasopharyngeal swabs were collected at 6 and 12 months of age to show the impact of the 2-month dose on pneumococcal carriage.
Results
Based on analysis to date of 1152 of 3200 swabs, vaccine-type carriage was low. In unvaccinated participants, PCV10 and PCV13-type carriage were 5.1% and 10.4% at 6 months, and 8.3% and 12.0% at 12 months, respectively. A dose of PCV10 transiently reduced vaccine-type carriage at 6 months of age (3/178 [1.7%] versus 18/355 [5.1%]).
Conclusions
With the exception of the PCV10 group at 6 months of age, both vaccine-type and non-vaccine-type carriage rates were similar among PCV10-vaccinated participants, PCV13-vaccinated participants and unvaccinated controls at 6 and 12 months of age. Based on preliminary data, a single dose of PCV at 2 months of age does not appear to reduce pneumococcal carriage during the first year of life in this population.
EVOLUTION OF INVASIVE PNEUMOCOCCAL DISEASE (IPD) BURDEN AND THE POTENTIAL EFFECT OF UNIVERSAL PNEUMOCOCCAL VACCINATION IN BOGOTÁ, COLOMBIA (ID 740)
Abstract
Background
IPD is the leading cause of infectious death worldwide. The universal pneumococcal vaccine in children has reduced IPD burden in several countries around the world. Importantly, after vaccine introduction, different pneumococcal serotypes are more frequently identified. However, in Bogotá-Colombia, there are scares data about the effect of the pneumococcal vaccine and its burden. Therefore, this study aims to provide novel data
Methods
This is a retrospective analysis of the pneumococcal reports of a surveillance program during the last 15 years in Bogotá, Colombia. S. pneumoniae were identified in each hospital and then characterized and serotyped in a governamental centralized laboratory. Descriptive statistics were used
Results
A total of 2605 cases were analyzed. Most common serotypes were 14 (7.11%), 1 (3.13%) 6A and 6B (2.71%) before 2010. Between 2011-2019, the most common serotypes changed to 19A (14.9%), 3 (8.06%) and 6C (3.86%) (Figure-1). Importantly, there was no change in the incidence of invasive pneumococcal disease throughout the study

Conclusions
Most common serotypes were 14 (7.11%), 1 (3.13%) 6A and 6B (2.71%) before 2010. Between 2011-2019, the most common serotypes changed to 19A (14.9%), 3 (8.06%) and 6C (3.86%) (Figure-1). Importantly, there was no change in the incidence of invasive pneumococcal disease throughout the study
REDUCTION OF PNEUMONIA WITH EMPYEMA AFTER THE INTRODUCTION OF THE PNEUMOCOCCAL CONJUGATE VACCINE IN EL SALVADOR (ID 756)
Abstract
Background
PCV has effectiveness in pneumonia with empyema (PE), PCV was introduced in 2010 (PCV7 3 + 1) and 2011 (PCV13 2 + 1) with coverage of 94%. In the pre vaccinations era (2002-2009) 6 cases/year were reported, in the post PCV7 stage there was no reduction in cases and in 2013 the reduction was 67%. The objective was to describe the impact of the PCV13 on the reduction of pneumococcal disease in the National Children's Hospital.
Methods
Analytical cross-sectional retrospective study. The total hospitalized patient with PE were reviewed (2002- 2018) with positive culture in pleural fluid to pneumococcus, epidemiological variables and vaccination status, 82 cases were reviewed.
Results
In the prevaccine period 49 cases were reported (2002-2009), in the post implementation (2010-2012) 28 cases, and from 2013-2018 5 were described, 4 are serotypes not contained in the vaccine (11, 13, 12A) and a case of 19F in a 12-year-old child with a single dose of PCV13.
Conclusions
After 9 years of PCV use in El Salvador there has been a decrease in cases of PE
MEDICAL RISK GROUPS BENEFIT FROM INDIRECT EFFECTS OF PCV13: A NATION-WIDE NORWEGIAN COHORT STUDY (ID 763)
Abstract
Background
We investigated indirect effects of PCV13 use in the childhood immunization programme in Norway on the incidence of invasive pneumococcal disease (IPD) in medical risk groups.
Methods
Our cohort included all Norwegian inhabitants aged ≥5 years during 2009-2017. We linked hospital discharge diagnoses (ICD-10 codes) from the Norwegian Patient Register with IPD notifications from the Norwegian Surveillance System for Communicable Diseases (n=5535). The cohort was stratified in risk-groups based on ICD-10 codes. We calculated incidences and annual changes (1-incidence rate ratios) for the years since PVC13 introduction (2011).
Results
The annual change in PCV13-type IPD was similar between risk groups (Overall, 19% [95%CI 17-22]). Non-PCV13 IPD increased on average by 4% [2-6] yearly (range 2-4% between groups). The overall change in IPD was non-significantly lower in risk groups (2% [95%CI 0-4%]) compared to the no-risk group (7% [5-9%]) resulting from the higher non-PCV13 incidence (32/100,000 versus 4/100,000) and larger proportion of cases (median for 2009-2017: 72% and 50%, respectively).
Conclusions
Overall, the IPD incidence decreased after PCV13 introduction in childhood immunisation. The annual change was slightly lower in risk groups compared to the no-risk group due to the dominance of non-PCV13 cases among risk groups and its substantially higher incidence.
BURDEN OF DISEASE ASSOCIATED WITH PNEUMONIA AND INVASIVE PNEUMOCOCCAL DISEASE (IPD) IN INDIVIDUALS AGED 15 YEARS AND ABOVE IN SPAIN (ID 774)
Abstract
Background
The available burden of disease (BoD) studies in pneumococcal disease in Spain are limited to analyzing data restricted to specific geographical regions. This study assessed the clinical burden associated with streptococcus pneumoniae in Spain.
Methods
A retrospective study was conducted using Conjunto Mínimo de Datos-Hospitalización (CMBD-H), a publicly available database provided by the Spanish Ministry of Health covering approximately 90% of hospitalization episodes.
The study population consisted of individuals aged 15 years and older who were hospitalized with a diagnosis of pneumonia, bacteremia and meningitis due to Streptococcus Pneumoniae in 2015.
Results
In 2015, the estimated burden of pneumococcal disease potentially avoidable through vaccination represented 10,274 inpatient admissions including 9,015 cases of pneumonia, 309 cases of meningitis and 950 cases of septicemia. Additionally, 867 deaths were registered during these hospitalization episodes. The information available in CMBD-H only captures individual hospitalization episodes and cannot capture per patient hospitalization, therefore it could be used as an approximation to estimate disease incidence, but not real incidence, which is a limitation of the study.
Conclusions
Pneumococcal disease still contributes to substantial morbidity and mortality among individuals aged 15 years and older in Spain.
ARE 10V CONJUGATED PNEUMOCOCCAL AND INFLUENZA VIRUS VACCINES- PROTECTIVE FACTORS FOR SEVERE PNEUMONIA? (ID 804)
Abstract
Background
Pneumonia plays an important role in children’s morbidity and mortality. In Brazil, epidemiological and social changes occurred concomitantly with the universal introduction of the 10-valent pneumococcal conjugate vaccine.This study identified risk factors for pneumonia.
Methods
A hospital-based, case-control study involving incident cases of pneumonia in children aged 1-59 months was conducted between October 2010 and September 2013 at a tertiary hospital in northeastern Brazil. The diagnosis of pneumonia was based on the WHOcriteria.The control group children admitted to the day-hospital ward for elective surgery. Children with comorbidity were excluded. The risk factors finvestigated were among those classified the WHO as definite, likely and possible. A multivariate analysis was performed.
Results
The study evaluated 452 children in the case group and 407 in the control group. Household crowding (OR=2.08) and not having been vaccinated against the influenza virus (OR=3.7) were the only factors found to increase the likelihood of pneumonia. Being male constituted a protective factor (OR=0.52).
Conclusions
The risk factors for pneumonia underwent changes that were probably associated with the expansion of the vaccination program and social improvements; however, these proved insufficient to overcome inequalities.The possible protection provided by the influenza vaccine needs to be evaluated against new etiological studies.
PNEUMOCOCCAL CONJUGATE VACCINE IS EFFECTIVENESS AGAINST HYPOXIC PNEUMONIA IN LAOS, MONGOLIA AND PAPUA NEW GUINEA: A NOVEL CASE-CONTROL VARIANT STUDY (ID 852)
- Fiona M. Russell, Australia
- Cattram D. Nguyen, Australia
- Rupert Weaver, Australia
- Christopher C. Blyth, Australia
- Jocelyn Chan,
- Claire Von Mollendorf, Australia
- Kate Britton, Australia
- David A. Dance, Laos
- Rebecca Ford, Papua New Guinea
- Jana Y. Lai, Australia
- Tuya Mungan, Mongolia
- Paul N. Newton, United Kingdom
- Kim E. Mulholland, Australia
- William Pomat, Papua New Guinea
- Keoudomphone Vilivong, Laos
- Anonh Xeuatvongsa, Laos
Abstract
Background
We describe a novel approach to determine PCV13 effectiveness (VE) against hypoxic pneumonia in children admitted with pneumonia in Lao PDR (Laos), Mongolia and Papua New Guinea (PNG).
Methods
A 3-5 year prospective hospital-based observational study of children <59 months admitted with pneumonia was undertaken. Pneumonia was defined using the 2013 WHO definition. Hypoxia was defined as an oxygen saturation <90% in room air or requiring oxygen supplementation during hospitalisation. PCV13 status was determined by written record. VE was calculated using logistic regression comparing the odds of hypoxia between vaccinated and undervaccinated pneumonia cases. To handle potential confounders a propensity score (PS) analysis using inverse probability of treatment weighting (IPW) was used. In Laos, multiple imputation (MI) analysis was undertaken for missing data.
Results
The VE against hypoxic pneumonia were: in Laos, unadjusted 23% (95% CI: -9, 46%; p=0·14), PS adjusted IPW 37% (6, 57%; p=0·02), MI adjusted 35% (7, 55%; p=0·02); in Mongolia, unadjusted 33% (26, 40%; p<0.001), PS adjusted IPW 33% (16, 47%; p<0.001); and in PNG, unadjusted 6% (-15, 24%; p=0.532), PS adjusted IPW 36% (17, 51%; p=0.001).
Conclusions
Our novel approach shows that PCV13 is effective against hypoxic pneumonia. PCV13 will contribute to reducing child mortality.
DYNAMICS OF BACTERIAL CARRIAGE DURING 12 YEARS POST PNEUMOCOCCAL CONJUGATE VACCINE (PCV) INTRODUCTION IN THE NETHERLANDS (ID 871)
Abstract
Background
Control of pneumococcal carriage is key to prevention of pneumococcal disease. We assessed the impact of PCV on carriage prevalence of Streptococcus pneumoniae(Sp) serotypes but also of Haemophilus influenzae(Hi), Staphylococcus aureus(Sa), Moraxella catarrhalis(Mc) and Group A Streptococcus(GAS).
Methods
Cross sectional observational study on carriage prevalence, determined by culture of nasopharyngeal swabs, in 24-month-old children performed in 2018/2019. Data were compared to studies performed in 2009, 2010, 2012 and 2015.
Results
Since PCV7 introduction in 2006 and change to PHiD-CV10 in 2011, overall carriage prevalence has decreased for Sp (66 to 49%) but increased for Hi (52 to 73%), Mc (59 to 83%), Sa (5.6 to 10%) and GAS (2.2 to 6.1%). Pneumococcal PHiD-CV10-type serotypes have disappeared. Initially carriage rate of NVT serotypes 19A, 11A, 10A and 23A increased but has declined again; 6C, 15A, 15B and 23B prevalence is still increasing. In 2018, 6C, 19A, 23B and 15B were the predominant serotypes in carriage and invasive disease in children <5 years of age.
Conclusions
Twelve years post-PCV introduction the decline in pneumococcal carriage has stabilized, but changes in frequency and nature of carried NVT Sp, Hi, Sa, Mc and GAS continued and should be considered for invasive and respiratory infection predictions.
INVASIVE NON-VACCINE SEROTYPE DISEASE AFTER THE INTRODUCTION OF PCV13 IN EL SALVADOR (ID 889)
Abstract
Background
PCV 7 and 13 were introduced in El Salvador in 2010 and 2011 respectively, with a decrease of more than 85% of invasive infections. However, an increase in replacement strains has been found that behaves highly invasive with high lethality.
Methods
60 patients admitted to Hospital de Niños Benjamin Bloom from January 2011 to December 2017 with invasive infections due to serotypes (S) not contained in the vaccine were described.
Results
60 records of children under 14 years of age with invasive pneumococcal infection and positive cultures in sterile fluids were reviewed, empyema pneumonia 20 (33%); meningitis 11 (18%); sepsis 12 (20%) and septic shock 12 (20%); pneumonia 5 (8%). Majorly responsible replacement serotypes: 10 A, 15 A, 11, 12 A, 18 A, 10 B, 8, 14 F, 15 C, 12 A. of the total of 33 (55%) cases. 30% overall mortality.
Conclusions
The Valente 13 vaccine has been highly effective in El Salvador, but non-vaccine serotypes have produced Invasive Infections with a high percentage of mortality. It is important epidemiologically to report the phenomenon of replacement.
PRELIMINARY RESULTS OF PCV-13 IMPACT STUDY IN HIV-INFECTED PATIENTS IN INDONESIA (ID 893)
Abstract
Background
Reduction in vaccine-type S. pneumoniae carriage can potentially reduce the risk of pneumococcal infection. However, the efficacy of the vaccine in HIV-infected individuals are still debatable.
Methods
HIV-infected patients (n=50) with mean age of 11.3 (6.4-16.8) years old in Jakarta were randomly enrolled into two groups. One group received a single dose of PCV-13 while the control group did not receive PCV-13. Nasopharyngeal (NP) swabs were collected to evaluate the impact of PCV-13 vaccination on S. pneumoniae colonization. The study will follow both groups until 18 months after the vaccination to find the difference of colonization and pneumococcal antibody in both groups at 6, 12, and 18 months period. This abstract reported the first phase of our study.
Results
We found 46% of NP swabs collected at initial phase were positive for S. pneumoniae with no significant difference in carriage rates between control and treatment group (p=0.917). We evaluate potential risk factors and found that school attendance significantly associated to colonization of S. pneumoniae in treatment group with p value = 0.015.
Conclusions
This result provides baseline data for pneumococcal vaccine evaluation in HIV-infected patients. We found 46% subjects were carrier for S. pneumonia and it was at the similar rate as previous studies.
DECLINING TRENDS IN MENINGITIS AMONG CHILDREN LESS THAN FIVE YEARS OF AGE FOLLOWING THE INTRODUCTION OF THE PNEUMOCOCCAL CONJUGATE VACCINES IN WEST AND CENTRAL AFRICA (ID 973)
Abstract
Background
By 2015, pneumococcal conjugate vaccines (PCVs) had been introduced into the infant immunization programmes of most countries in West and Central Africa. We modelled the trends in meningitis cases and deaths among children before and after PCV introduction.
Methods
A total of 36,901 children under 5 years of age with suspected meningitis were enrolled at sentinel hospitals across 10 West and Central African countries between 2010 and 2016 through the Paediatric Bacterial Meningitis (PBM) Surveillance Network . To assess disease and mortality trends before and after PCV introduction, we applied interrupted time-series models and random effects meta-analysis.
Results
Across the sub-regions, there was a decline of 35% (95% CI 2-57%, p=0.04) in annual suspected meningitis cases and 26% (95% CI 3-44%, p=0.03) in laboratory confirmed meningitis in the post vs. pre-PCV period. Likewise, there was a decreased trend in mortality among suspected meningitis cases (33% decline, 95% CI -23-52%, p=0.27) post PCV introduction. There was considerable heterogeneity among countries with the larger and more precise reduction estimates in countries with >2 years post-PCV surveillance.

Conclusions
We observed significant declines in suspected and confirmed pediatric meningitis across the sub-regions following PCV implementation. Continued monitoring, particularly in countries with more recent PCV introduction is needed.
MONITORING VACCINE IMPACT ON COMMUNITY CARRIAGE IN NEPAL REVEALS CHANGES IN THE CIRCULATING POPULATION OF PNEUMOCOCCAL SEROTYPES AND ANTIMICROBIAL RESISTANCE GENES (ID 977)
- Sonu Shrestha, United Kingdom
- Rama Kandasamy, Australia
- Susana Camara,
- Merryn Voysey, United Kingdom
- Madhav C. Gautam, Nepal
- Meeru Gurung, Nepal
- Katherine Gould,
- Stephen Thorson, Nepal
- Imran Ansari, Nepal
- Shrijana Shrestha, Nepal
- Maria D. Knoll, United States of America
- David Murdoch, New Zealand
- Dominic Kelly, United Kingdom
- Jason Hinds, United Kingdom
- Andrew J. Pollard, United Kingdom
Abstract
Background
Community carriage of pneumococcal serotypes in children was assessed pre- and post-PCV10 introduction in Nepal to monitor pneumococcal vaccine impact. Molecular serotyping by microarray enabled detection of multiple-serotype carriage plus non-encapsulated pneumococcal lineages, related Streptococcus species and selected antimicrobial resistance genes.
Methods
Nasopharyngeal swabs were collected from healthy Nepalese children in 2014-15 (pre-PCV10) and 2017-18 (post-PCV10). DNA was extracted from plate sweeps of 1,241 and 1,445 swab cultures for pre- and post-vaccine periods respectively and analysed by Senti-SP molecular serotyping microarray.
Results
Comparing carriage among children pre- and post-PCV10, there was a decrease in PCV10 serotype carriage (37% vs 17%) and an increase in non-vaccine serotype carriage (67% vs 73%). There was no change for non-encapsulated pneumococcal lineages (16% vs 16%), an increase in related Streptococcal species (22% vs 25%) and an increase in detection of antimicrobial resistance genes (65% vs 74%). Multiple pneumococcal serotype carriage decreased (24% vs 16%) and multiple carriage including non-encapsulated pneumococci and related Streptococcal species also decreased (45% vs 41%).
Conclusions
Introduction of PCV10 in Nepal has resulted in a decrease in vaccine type carriage within two years. However, increases in carriage of non-vaccine types as well as antimicrobial resistance genes and related Streptococcal species were observed.
INTRODUCTION OF TEN VALENT PNEUMOCOCCAL VACCINE IMPARTS INDIRECT PROTECTION TO CHILDREN IN A RURAL POPULATION IN PAKISTAN (ID 978)
Abstract
Background
Ten-valent pneumococcal vaccine was introduced in Pakistan’s immunization program in 2012 as 3+0 schedule without catchup immunization.
Methods
From 2014 to 2018, children were randomly selected from a line listing in two union councils of Matiari, Pakistan. Nasopharyngeal swabs were collected using standard WHO guidelines and processed at Infectious Disease Research Laboratory at Aga Khan University in Karachi. Serotypes for pneumococcal isolates were deduced using published sequential multiplex PCR assays.
Results
Of 3140 children enrolled, pneumococcal isolates were detected in 2370(75%). VT carriage decreased from 11.4 to 8.2 % (p-value=0·031) in vaccinated group and from 19.3 to 12.7% (p-value= 0·003) in non-vaccinated group. On average VT carriage decreased from 16·1% to 9·6% (p-value<0·001). Most significant decline was seen in serotypes 6B, 9V/9A and 19F. Proportion of fully immunized children changed from 41·0% to 68·4% (p-value<0·001). Direct effect of the vaccine was calculated to be 33·5% (95% CI 19·5%-55·0%) and indirect effect to be 44·1% (95% CI 28·1% -56·6%).

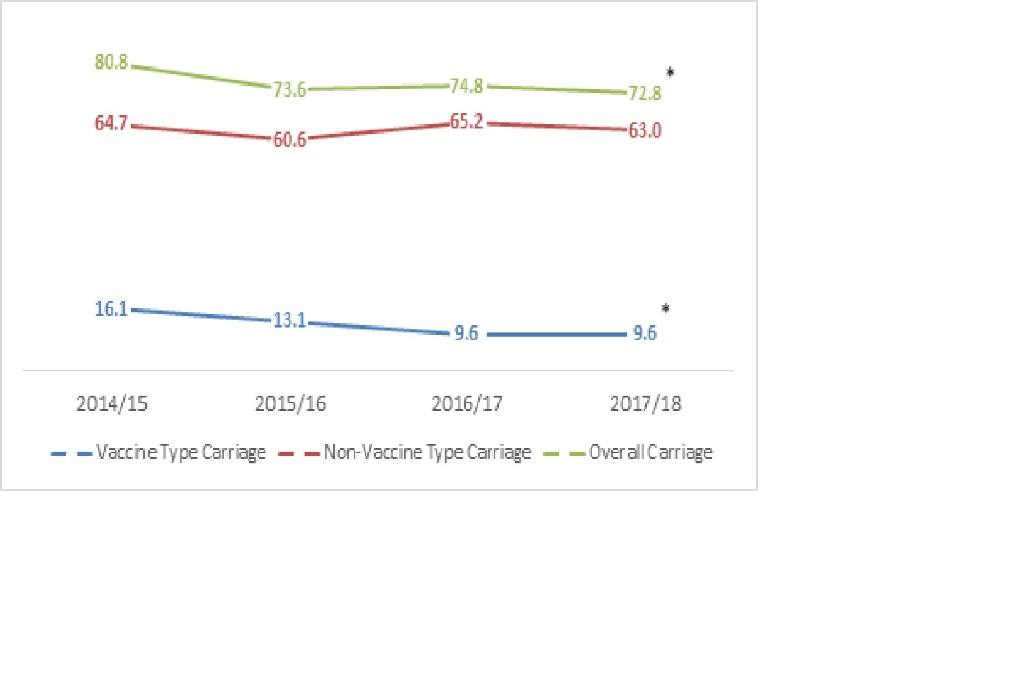
Conclusions
We saw substantial decline in pneumococcal carriage in young children in a rural community which was seen in both vaccinated and unvaccinated groups. This is indicative of herd immunity and will hopefully translate to decrease in pneumococcal disease burden in population.
HIGH CARRIAGE PREVALENCE OF ANTIMICROBIAL-NON-SUSCEPTIBLE PNEUMOCOCCI IN CHILDREN WITH RADIOLOGICALLY CONFIRMED PNEUMONIA THREE YEARS AFTER 10-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV10) INTRODUCTION IN MOZAMBIQUE (ID 996)
- Benild Moiane, Mozambique
- Fernanda Lessa, United States of America
- Sergio Massora, Mozambique
- Helio B. Mucavele, Mozambique
- Alberto Chauque, Mozambique
- Fabiana Pimenta, United States of America
- Cynthia G. Whitney, United States of America
- Benard Beall, United States of America
- Nelson Tembe, Mozambique
- Maria G. Carvalho, United States of America
- Betuel Sigauque, Mozambique
Abstract
Background
Antimicrobial resistance (AR) is a global problem, and drug-resistant pneumococci are a serious AR threat. We evaluated carriage of antimicrobial non-susceptible pneumococci in children with X-ray confirmed pneumonia (XRCP) 3 years after PCV10 introduction in Mozambique.
Methods
We collected nasopharyngeal swabs from children who were age-eligible for PCV and hospitalized with XRCP at 3 hospitals (2 urban, 1 rural) in southern Mozambique between October 2015-May 2016. Specimens were cultured for pneumococci; isolates underwent serotyping by Quellung and antimicrobial susceptibility testing using broth microdilution. Non-susceptible isolates had intermediate or full resistance based on CLSI 2018 breakpoints; multi-drug non-susceptible (MDNS) isolates were non-susceptible to ≥3 antimicrobial classes.
Results
Pneumococci were detected in 90 (42.1%) of 214 children with XRCP. Of those, 28.9% (26/90) and 81.1% (73/90) carried PCV10-type and penicillin-non-susceptible pneumococci, respectively. Among 29 (32%) children colonized with MDNS-pneumococci, serotypes 23F (27.6% [n=8]) and 19F (17.2% [n=5]) were most prevalent. PCV10-types were more associated with penicillin-non-susceptibility (100% vs. 73.4%, P=0.002) and MDNS (69.7% vs. 17.2%, P<.001) than non-PCV10-types. Non-susceptibility to 3rd generation cephalosporins was found in two 19F isolates.
Conclusions
High prevalence of penicillin-non-susceptible and MDNS-vaccine-type pneumococcal carriage among children with pneumonia post-PCV10 introduction in Mozambique is concerning and suggests new prevention strategies are needed.
SEROTYPE-SPECIFIC PNEUMOCOCCAL CARRIAGE DENSITY AMONG HEALTHY NEPALESE CHILDREN POST-PCV10 INTRODUCTION (ID 1047)
- Sonu Shrestha, United Kingdom
- Rama Kandasamy, Australia
- Madhav C. Gautam, Nepal
- Meeru Gurung, Nepal
- Stephen Thorson, Nepal
- Imran Ansari, Nepal
- Katherine Gould,
- Michael J. Carter, United Kingdom
- Dominic Kelly, United Kingdom
- David Murdoch, New Zealand
- Andrew J. Pollard, United Kingdom
- Jason Hinds, United Kingdom
- Shrijana Shrestha, Nepal
Abstract
Background
The density of pneumococcal carriage provides an insight into the dynamics of transmission, colonisation and vaccine effect. We aimed to measure serotype-specific carriage density 2-3 years after 10-valent pneumococcal conjugate vaccine (PCV10) introduction, using qPCR and bacterial DNA microarray.
Methods
Nasopharyngeal swabs were collected from healthy Nepalese children from the Kathmandu Valley between April 2017 and August 2018. DNA was extracted from the swab media and qPCR performed for pneumococcal autolysin (lytA). Swab media were also plated on blood agar, incubated overnight, and plate sweeps collected. DNA was extracted from plate sweeps and molecular serotyped using the Senti-SPv1.5 microarray (BUGS Bioscience, UK).
Results
1264 swabs were collected and analysed by both microarray and qPCR. The mean density of PCV10 serotypes was significantly higher than non-PCV10 serotypes (10^3.9 vs 10^3.4 copies/ml, p=0.004). Serotypes 1 (10^4.6 copies/ml, 95% CI 10^3.4-5.9) and 6B (10^4.6 copies/ml, 95% CI 10^3.9-5.3) had the highest mean density. Serotypes 4 (10^2.8 copies/ml, 95% CI 10^1.1-4.5) and 9N (10^2.9copies/ml, 95% CI 10^2.1-3.7) had the lowest mean carriage density.
Conclusions
Serotype 1, which causes the greatest proportion of invasive pneumococcal disease in this setting, was found to have the highest carriage density. Further evaluation of the PCV10 impact on carriage density is needed.
IMPACT OF THE 13-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV13) ON INVASIVE PNEUMOCOCCAL DISEASE IN ALASKAN ADULTS (ID 1075)
Abstract
Background
Background: In April 2010, PCV13 was introduced statewide for children in Alaska. To evaluate the impact of PCV13 on IPD in adults, we assessed IPD rates by serotype, prior to and 8 years after PCV13 introduction.
Methods
Pneumococcal sterile site isolates, reported through state-wide surveillance, were serotyped using standard methods. We defined the pre-PCV13 time period as 2005-2008 and the post-PCV13 time period as 2011-2018. We compared proportions using chi-squared or Fisher’s exact test. Population denominators were obtained from the Alaska Department of Labor website.
Results
Among all adults 18-49 years, IPD rates decreased from 13.6(pre) to 8.9(post) per 100,000/yr (P<.001); PCV13 serotype IPD decreased (4.8 to 1.7,P<.001). Among Alaska Native adults 18-49 years, IPD rates decreased (41.1 to 25.5,P<.001); PCV13 serotype IPD decreased (9.0 to 3.7,P=.006); rates of non-PCV13 serotype IPD decreased (29.9 to 20.4,P=.016). Among adults >50 years, overall IPD rates did not change significantly; PCV13 serotype IPD decreased (14.1 to 7.2,P<.001); rates of non-PCV13 serotype IPD increased (16.7 to 23.1,P=.002) from pre- to post-PCV13 time periods.
Conclusions
Eight years after PCV13 introduction, overall IPD and PCV13-serotype IPD rates decreased in Alaskan adults 18-49. We observed an increase in non-PCV13 serotype IPD rates among adults 50+ when compared with 2005-2008.
IMPACT OF THE 13-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV13) ON INVASIVE PNEUMOCOCCAL DISEASE IN ALASKAN CHILDREN (ID 1076)
Abstract
Background
In April 2010, PCV13 was introduced statewide in Alaska. To evaluate the impact of PCV13 on IPD in children, we assessed IPD rates by serotype, prior to and 8 years after PCV13 introduction.
Methods
Pneumococcal sterile site isolates, reported through state-wide surveillance, were serotyped using standard methods. We defined the pre-PCV13 period 2005-2008 and post-PCV13 period 2011-2018. We compared proportions using chi-squared or Fisher’s exact test. Population denominators were obtained from the Alaska Department of Labor website.
Results
Among Alaska children <5 years, PCV13 serotypes comprised 62%(78/126) of IPD isolates in the pre-PCV13 period and 19%(18/95) in the post-PCV13 period. Among all Alaska children <5 years, IPD rates decreased 64% from 60.9(pre) to 22.2(post) per 100,000/yr (95%CI:52%-72%); PCV13 serotype IPD decreased 89% from 37.7 to 4.2 (95%CI:81%-94%). Among Alaska Native children <5 years, IPD rates decreased 64% from 149.2 to 54.1 (95%CI:49%-74%); PCV13 serotype IPD decreased 89% from 87.0 to 10.0 (95%CI:78%-94%). Rates of non-PCV13 serotype IPD did not change significantly. Similar declines were observed among both rural and urban children; PCV13 IPD rates declined 91% (95%CI:80%-96%) and 86% (95%CI:70%-94%), respectively.
Conclusions
Eight years after PCV13 introduction, overall IPD and PCV13-serotype IPD rates decreased in Alaska children <5 years when compared with 2005-2008.
CONSISTENCY OF VACCINATION HISTORIES OBTAINED FROM MEDICAL RECORDS OR CAREGIVER RECALL IN NEPAL. (ID 1135)
- Sarah Kelly, United Kingdom
- Merryn Voysey, United Kingdom
- Meeru Gurung, Nepal
- Peter J. O'Reilly, United Kingdom
- Stephen Thorson, Nepal
- Bhishma Pokhrel, Nepal
- Ganesh Shah, Nepal
- Imran Ansari, Nepal
- Maria D. Knoll, United States of America
- Dominic Kelly, United Kingdom
- David Murdoch, New Zealand
- Andrew J. Pollard, United Kingdom
- Shrijana Shrestha, Nepal
Abstract
Background
PCV status, necessary for assessing vaccine impact, is often incomplete or unknown. For children admitted with pneumonia in Kathmandu, Nepal, we compared vaccine histories from caregivers vs medical records to determine if these provided similar estimates of coverage.
Methods
Between 2016-2019, patients aged 6 months to 14 years admitted with pneumonia at Patan Hospital, enrolled into a pneumococcal carriage study had their number of PCV doses collected by caregiver recall and/or from hospital medical records. Records, created at birth, are updated when they receive routine vaccinations there; for vaccinations administered elsewhere, vaccine history is obtained from caregivers during any hospital admission. Cases were excluded from analyses if both caregiver recall and medical records were cited as the source.
Results
Of the 1,603 inpatients enrolled, 1,201 (80%) had data from either caregiver recall or medical records. PCV coverage (3 doses) was higher for caregiver recall than medical records (43% vs 35%; p=0.03), while Hib vaccine coverage was similar (91% vs 87%; p=0.16).
Conclusions
Although caregiver recall provided statistically higher estimates of vaccine coverage than medical records, the estimates were generally similar. Medical records may be incomplete (underestimate) and caregiver recall may have recall bias (overestimate); the truth may be in between.
EPIDEMIOLOGY OF PNEUMOCOCCAL PNEUMONIA IN POST-PNEUMOCOCCAL VACCINATION ERA IN A PEDIATRIC HOSPITAL IN ARGENTINA (ID 1212)
Abstract
Background
In January 2012, PCV13 was introduced into immunization program in Argentina, 2+1 schedule for <2 years. The aims of this study were to evaluate the impact of PCV13 on Pneumococcal Pneumonia (PP) burden, and to describe epidemiological-clinical pattern of PP during the post-vaccination period.
Methods
PP discharge rates per 10,000 hospital discharges were compared between the pre-vaccination period 2007-2011 (preVp), the year of intervention (2012) and the post-vaccination period 2013-2019 (postVp).
Results
Reduction in PP discharge rates was observed in patients <5 years (34.7%) in 2012 and 15.3% in postVp; these changes were not significant. Of 78 PP cases, 53.8% were male; 21.8% <2 years; 29.5% (23/78) had a complete PCV13 schedule (1 immunosuppressed case); 34.6% (27/78) had underlying diseases; 69.2% had complications (pleural effusion 69.2%, necrotizing pneumonia 10.3%, pneumothorax 9.0%, lung abscess 5.1%). Other clinical manifestations combined with PP were: sepsis 12.8%, meningitis 1.3% and peritonitis 1.3%. No case died. PP was more associated with age ≥84 months between 2012-2015 vs 2016-2019 [OR 4.68(1.09-23.10)].
Conclusions
After PCV13 introduction a reduction in PP discharge rates was observed in hospitalized children <5 years. PP cases affected older children
in the first years posV.
IMPACT AND EFFECTIVENESS OF THE 10-VALENT PNEUMOCOCCAL CONJUGATE VACCINE ON INVASIVE PNEUMOCOCCAL DISEASE IN THE NETHERLANDS (ID 443)
Abstract
Background
In 2011, 10-valent pneumococcal conjugate vaccination (PCV10) was introduced in the Dutch National Immunization Program, after five years of PCV7. We report on the impact and effectiveness of PCV10 in the Netherlands.
Methods
We included IPD cases reported from June 2004 up to May 2019 to our surveillance system. We estimated vaccine effectiveness using the indirect cohort method in children eligible for PCV10. We assessed impact in four age groups by comparing IPD incidences in June 2017-May 2019 and June 2009-May 2011 (before switch to PCV10).
Results
Effectiveness of at least two doses of PCV10 was 90% (63%;97%) against PCV10-type IPD and 29% (-177%;82%) against 19A IPD. Overall IPD incidence decreased by 17% (-5%;35%) in children <5 years, 26% (9%;40%) in 5-49 year-olds, 6% (-10%;19%) in 50-64 year-olds and 2% (-9%;11%) in 65+ year-olds. 19A IPD incidence decreased by 17% (-41%;51%) in children <5 years and by 4% (-103%;55%) in 5-49 year-olds, and increased by 77% (12%;180%) in 50-64 year-olds and by 35% (-1%;84%) in 65+ year-olds.
Conclusions
Despite good effectiveness on vaccine serotypes, the switch to PCV10 has had limited impact on further reducing overall IPD burden after five years of PCV7. Serotype 19A IPD incidence is slowly increasing in older adults.
CHANGES IN SEROTYPE 3 (ST3) INVASIVE PNEUMOCOCCAL DISEASE (IPD) AFTER 13-VALENT CONJUGATE VACCINE (PCV13) INTRODUCTION IN SOUTHERN ONTARIO (ID 506)
Abstract
Background
We asked whether the epidemiology of ST3 IPD changed after implementation of infant PCV13 (2010) and immunosuppressed 50+ (2014) programs.
Methods
Our research network conducts population-based surveillance for IPD in Toronto/Peel, Ontario. We assessed incidence of IPD due to ST3 and compared the characteristics of IPD due to ST3 and other STs (OST) pre- (2001-2009) and post- (2013-2018) PCV13 introduction.
Results
8183 cases of IPD occurred from 2001-present (733 ST3). Figure shows ST3 incidence: compared to pre-PCV13, the incidence post-PCV13 was lower in 65+ was lower (IRR 0.66 (0.53-0.81), P<0.001), but higher in 50-64yrolds (IRR 1.45 (1.14-1.85), P=0.003).

Post-PCV13 compared to pre-PCV13, the proportion of IPD in homeless persons increased in both ST3 and OST (1.5-6.6% and 1.8-5.4%), while the proportion of cases with any underlying chronic illness and immunosuppression trended down in ST3 (67-61% and 25-21%), but increased significantly in OST (63-69%, 29-34%, both P<0.0012). The ICU admission and case fatality rate of ST3 declined (45-40%, P=NS and 29-21%, P=0.03), while those due to OST did not change (25-27% and 17-17%, P=NS).
Conclusions
In our population, pediatric PCV13 program implementation was temporally associated with a decline in ST3 disease in older adults but an increase in 50-64yrolds; case fatality also declined.
EARLY IMPACT OF INTRODUCING A TEN-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV10) ON PNEUMOCOCCAL CARRIAGE IN NIGERIA (ID 652)
Abstract
Background
As Nigeria transitions from Gavi-support to self-financing of the pneumococcal conjugate vaccine (PCV), decisions on vaccine cost effectiveness should be based on local vaccine impact data. Herd immunity against carriage is a major contributor to PCV impact. So, carriage surveys are a useful option for impact assessments in the absence of disease surveillance systems.
Methods
We conducted nasopharyngeal carriage surveys (2017-2019) according to WHO guidelines among randomly selected residents of two locations (urban and rural) in Nigeria. PCV10 was introduced in 2016 in these locations and reached a modest coverage of 37% and 61% by 2019. Carriage prevalence ratios (PR) before and after PCV10 introduction were estimated using log-binomial regression.
Results
There was a 38% (PR-0.62 [95%CI:0.53-0.72]) and 21% (PR-0.79 [95%CI:0.66-0.94]) reduction in carriage of vaccine serotypes respectively among vaccine-target (<5years) and non-target) (5+ years) groups, mostly due to serotypes 19F, 23F and 6B. Carriage of non-vaccine serotypes increased by 28% (PR-1.28 [95%CI:1.15-1.42]) and 31% (PR-1.31 [95%CI:1.20-1.43]) respectively in these groups; serotypes 6A, 19A, 34, 16F and 11A were prominent.
Conclusions
Within three years of PCV10 introduction, we found early evidence of direct and indirect PCV effects on vaccine serotype carriage as well as serotype replacement in carriage.
INVASIVE PNEUMOCOCCAL DISEASE IN THE GAMBIA: TRENDS IN SEROTYPE PREVALENCE PRE AND POST PCV INTRODUCTION (ID 661)
Abstract
Background
The introduction of PCV7 in August 2009 and PCV13 in May 2011 in The Gambia resulted in decline of invasive pneumococcal disease (IPD) incidence by 55% in the Eastern region although changing serotypes are emerging.
Methods
We retrospectively compared disease and serotype prevalence pre-PCV (January 2005-December 2009) and post-PCV (January 2012 - December 2015) periods for IPD in the Western region of the country
Results
Out of 12,454 blood and 979 CSF cultures analysed, 6.8% (847/12454) blood and 6.9% (68/979) CSF were clinically significant pathogens. The prevalence of IPD for suspected bacteraemia was 1.8% (218/12454) constituting 25.8% (218/846) of all confirmed cases and 3.9% (38/979) for meningitis constituting 55.9% (38/68). When compared by vaccine period, a significant drop across all age groups post-PCV was found, decreasing from 32.4% (160/494) to 16.5% (58/352) for bacteraemia and from 67.3% (33/49) to 26% (5/19) for meningitis.
Serotype data was available for 86.3% (221/256) and decrease in PCV13 vaccine serotypes from 62.2% (120/193) to 41.3% (26/63). Concurrently increase in non-vaccine serotypes from 24.9% (48/193) to 42.8 % (27/63) was found with 12F accounting for 50%.
Conclusions
The PCVs have reduced IPD but serotype replacement is noted warranting surveillance and more intervention.
Long-term Impact of Pneumococcal Conjugate Vaccine (PCV) on Antibiotic Resistant Invasive Pneumococcal Disease (IPD) in the United States (ID 892)
- Tamara Pilishvili, United States of America
- Ryan Gierke, United States of America
- Monica Farley, United States of America
- William Schaffner, United States of America
- Ann Thomas, United States of America
- Arthur Reingold, United States of America
- Lee Harrison, United States of America
- Ruth Lynfield, United States of America
- Kari Burzlaff, United States of America
- Susan Petit, United States of America
- Rachel Herlihy, United States of America
- Salina Torres, United States of America
- Bernard Beall, United States of America
Abstract
Background
PCVs have been recommended for U.S. children since 2000 and for adults aged ≥65 years since 2014. We evaluated impact of PCVs on antibiotic non-susceptible (NS) IPD.
Methods
IPD cases were identified through CDC’s Active Bacterial Core surveillance during 1998-2018. Isolates were serotyped and classified as PCV13 or non-vaccine type (NVT). We applied 2019 Clinical and Laboratory Standards Institute breakpoints to minimum inhibitory concentrations (using broth microdilution or whole genome sequencing) to classify isolates as NS to >1 antibiotic (NS-IPD) or to >3 drug classes (multi-drug-NS). Incidence rates (per 100,000) were calculated using U.S. Census Bureau population denominators.
Results
From 1998-1999 to 2017-2018, penicillin-NS IPD incidence decreased from 12 to 0.4 among children <5 years-old and from 5 to 0.8 among adults ≥65 years-old. Incidence of PCV13-type NS-IPD decreased among all ages, while incidence of NVT NS-IPD increased for all ages (Figure). In 2018, serotypes 19A (37%), 23A (13%) and 23B (13%) and serotypes 35B (42%), 19A (19%), and 15A (12%) accounted for most penicillin NS and multi-drug-NS IPD, respectively.

Conclusions
NS-IPD incidence decreased following 18 years of PCV use among children, driven by reductions in PCV serotypes. Increases in NVTs have started to erode PCV benefits on NS-IPD, especially among adults.
INCREASE IN FREQUENCY OF PNEUMOCOCCAL METABOLIC GENOTYPES CHARACTERISED BY ANTIMICROBIAL RESISTANCE AND ADAPTATIONS FOR COLONIZATION AFTER PCV13 INTRODUCTION IN BLANTYRE, MALAWI. (ID 416)
- Andrea Gori, United Kingdom
- Uri Obolski, Israel
- Todd D. Swarthout, Malawi
- Jose Lourenço, United Kingdom
- Caroline M. Weight, United Kingdom
- Jen Cornick,
- Dean Everett, Malawi
- Arox Kamng'ona,
- Thandie S. Mwalukomo,
- Martin C. Maiden, United Kingdom
- Neil French, United Kingdom
- Sunetra Gupta, United Kingdom
- Robert S. Heyderman, United Kingdom
Abstract
Background
Streptococcus pneumoniae naturally undergoes fluctuations in genotype. We previously showed a high residual prevalence of vaccine serotype (VT) pneumococci (18% in under 5’s) 7 years after PCV13 introduction in Malawi. In this context, we hypothesised the emergence and fixation of S. pneumoniae lineages with genetic traits conveying a competitive advantage in the nasopharyngeal niche.
Methods
1826 S. pneumoniae genomes were analysed from isolates collected during rolling cross-sectional carriage surveys in Blantyre, 4-7 years after PCV13 introduction. The metabolic core-genome includes 175 discrete metabolic genotypic profiles (metabolic types, MTs). Relative fitness was assessed by in-vitro growth and adhesive potential evaluated using Detroit 562 nasopharyngeal epithelial cells.
Results
High residual pneumococcal carriage is characterised by persistent MTs in VT (e.g. 3 and 23F) and emerging new MTs in non-VT (NVT; 38 and 23B). Increase in AMR MTs among 38 and 23B was observed. Emerging MTs show characteristic sequences of virulence genes and are characterised phenotypically by higher growth potential and propensity for better adherence to NP cell. We identified convergent evolution between MTs isolated in different countries, result of genetic bottlenecks.
Conclusions
This shift in metabolic genotypes, antimicrobial resistance and colonization adaptations may facilitate vaccine escape.
IMPACT OF 13-VALENT PNEUMOCOCCAL CONJUGATE VACCINE ON INVASIVE PNEUMOCOCCAL DISEASE IN MASSACHUSETTS CHILDREN, 2010/2017 (ID 495)
Abstract
Background
The 13-valent-pneumococcal conjugate vaccine (PCV13) replaced PCV7 in Massachusetts(MA) beginning in April,2010. We describe the current epidemiology of invasive pneumococcal disease (IPD) in MA children after 7 years of PCV13 use.
Methods
Cases of IPD in children <18 years of age were detected through an enhanced surveillance system. Parents/physicians/providers are interviewed for confirmation of demographic and clinical data. All Streptococcus pneumoniae from sterile body sites are submitted to Department of Public Health and serotyped by Quellung reaction.
Results
Incidence of IPD declined to 2.8/100,000 in 2017/18 (71% decline compared to prePCV13 baseline 9.8/100,000; incidence rate ratio 0.29,95%CI 0.24-0.32) mostly due to reduction in additional serotypes included in PCV13 (Figure). The most common clinical presentation was bacteremia(55%), followed by pneumonia(32%) and CNS disease(7%); 91(27%) children had >1 comorbidity [asthma(12%), hematologic malignancy(12%), prematurity(10%), sickle cell disease(10%)]. Mortality rate was 4.4%. Isolates from 302 (89%) were available for serotyping. Vaccine serotypes (VST) were identified in 98 (33%) IPD cases [19A(48%),7F(20%),3(18%), 19F(7%), 6A(3%), 14,18C,5(1% each). Serotypes 15BC(14%), 22F(12%) and 33F(12%) were the most common nonvaccine serotypes(NVST).

Conclusions
IPD declined >70% following 7-years of PCV13 use.NVSTs, specifically serotypes 15BC,33F and 22F are responsible for majority of the remaining disease which is disproportionately observed in children with comorbid conditions.
IMPACT OF 13-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV13) ON PEDIATRIC BACTEREMIC PNEUMOCOCCAL PNEUMONIA, 2010/2017 (ID 497)
Abstract
Background
Streptococcus pneumoniae (SPN) is a major cause of hospitalized community-acquired-pneumonia. We assessed the impact of PCV13 on the epidemiology of bacteremic pneumococcal pneumonia (BPP).
Methods
A population-based, enhanced surveillance for invasive pneumococcal disease among Massachusetts children is ongoing since 2001. SPN isolates from normally sterile body sites are sent to the Department of Public Health and serotyped using Quellung reaction. Parents/guardians/providers are interviewed to obtain demographic and clinical information.
Results
Three-hundred-ninety-one BPP cases were identified between 04/2002 and 03/2017. Incidence of BPP declined 79% in 2016/2017 compared to prePCV13 baseline (0.68/100,000 vs 3.35/100,000,rate ratio 0.19,95%CI 0.09-0.25)(Figure). One-hundred-six(26.0%) children had >1 comorbidity. Mortality rate was 2.6%. Serotypes 19A(37.5%), 7F(21.3%), 3(6.0%), 22F(3.7%) were the most frequent serotypes in the prePCV13-era; 19A(16.0%), 22F(11.7%),7F(11.7%),3(7.5%), and 33F(5.3%) were the most frequent serotypes in the postPCV13-era. Nonvaccine serotypes (NVST) were isolated in 42(19.4%) and 55(58.5%) of BPP cases in prePCV13 and postPCV13-eras, respectively. The incidence of NVST BPP increased from 0.36/100,000 to 0.56/100,000 annually in postPCV13 eras. Twenty-six(8.4%) isolates were penicillin-non-susceptible and 93(30.2%) were macrolide-non-susceptible.

Conclusions
In the post-PCV13 era, NVST are the most common cause of BPP, supporting a need for strategies to further reduce the burden of childhood pneumonia.
EFFECT OF PCV-10 ON INVASIVE PNEUMOCOCCAL DISEASE AMONG BANGLADESHI CHILDREN (ID 1010)
Abstract
Background
Bangladesh introduced PCV-10 in March 2015 using a 3+0 schedule. We evaluated PCV-10 effect on invasive pneumococcal disease (IPD) among children <5 years.
Methods
IPD surveillance is ongoing in four sentinel hospitals. Numbers of children with pneumococcus detected from a sterile site (IPD cases) were adjusted using number of febrile children tested with blood and/ CSF each year as the denominator. Data from pre-vaccine baseline (January 2012 to March 2015) and post-vaccine (April 2015 to September 2019) periods were compared to determine PCV-10 impact by age group. Serotypes in PCV-10 plus 6A were considered vaccine types (VT).
Results
We identified 543 children with IPD among 60,921 children tested during 2012 to September 2019. IPD rates among children <5 years decreased 61% (CI: 45.8-72.4%) between baseline (137/10,000 tested) and 2019 (53/10,000; Figure-A); VT IPD rates fell 71% (CI: 47.2-85.4%; 53 to 15/10,000). Among children 3-23 months, IPD declined 70% (CI: 54.9-81.9%) between baseline (207/10,000) and 2019 (63/10,000; Figure-B); VT rates dropped 87% [(CI: 65.2-96.5%); 81 to 10/10,000]. Nonvaccine-serotype IPD rates were stable.
Conclusions
PCV-10 introduction resulted in large reductions of overall and VT IPD among young children. In contrast to reports from elsewhere, serotype replacement is not yet evident in Bangladesh.
IMPACT OF 10-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV-10) ON PNEUMONIA HOSPITALIZATION RATE AMONG BANGLADESHI CHILDREN (ID 1134)
Abstract
Background
Bangladesh introduced PCV-10 in March 2015 with a 3+0 schedule. We assessed impact of PCV-10 on pneumonia and severe pneumonia among children <5 years.
Methods
Children <5 years were enrolled following WHO’s Invasive Bacterial Vaccine Preventable Disease surveillance criteria. January 2011 to March 2015 was considered as baseline and April 2015 to June 2019 as post-PCV era. PCV-10 impact was measured based on hospitalization rate (adjusted with all hospitalized patients) of pneumonia and severe pneumonia using (I) WHO case definitions and (II) physician’s diagnosis.
Results
Analysis using WHO’s case definition didn’t show any significant changes in pneumonia admissions among those <5 years or 3-23 months. Similarly, no impact was noted for admissions of physician-diagnosed pneumonia. However, physician-diagnosed severe pneumonia episodes decreased 82% (95% CI: 78.9%-84.2%) from 4.5%-0.8% among those <5 years and 76.1% (95% CI 69.8%-81.2%) from 4.4 -1% among children 3-23 months.
Conclusions
The lack of significant change for WHO-defined pneumonia and severe pneumonia or physician-diagnosed pneumonia may be due to a lack of specificity in the definitions, including many illnesses not caused by pneumococcal infection. Reduction in severe pneumonia admissions (among which more cases are expected to be bacterial), suggests a role of PCV-10 in reduction of pneumococcal pneumonia.
INVASIVE PNEUMOCOCCAL DISEASE AMONG CHILDREN IN GERMANY, TEN YEARS AFTER PCV13 INTRODUCTION (ID 899)
Abstract
Background
Childhood PCV vaccination was generally recommended in Germany in 2006. Here, we present data on invasive pneumococcal disease (IPD) cases following PCV introduction.
Methods
IPD in children in Germany has been monitored since 1997. Isolates were serotyped using the Neufeld Quellung reaction.
Results
In 2018-2019, the GNRCS received 102 IPD isolates from children <2 years, of which 14 had PCV13 serotypes. Two of these were from unvaccinated children, four from incompletely vaccinated children. This represents a 34% reduction compared to 2005/2006 (n=154), but an increase since 2011-2012 (n=75). However, the PCV13 proportion has decreased from 88% prior to vaccine introduction (2005-2006), to 69% at the introduction of higher-valent vaccines (2009-2010), to 14% in 2018-2019. Future vaccines PCV15 (25%) and PCV20 (46%) would increase coverage considerably. Residual PCV13 serotypes in 2018/2019 were 3 (n=5), 19F (n=4), 19A (n=2) and 6A, 14, 23F (n=1, each). Among all three age groups (0-1y, 2-4y, 5-15y), serotypes 3, 19F and 19A persist. Among non-vaccine serotypes, 10A (n=17) and 23B (n=12) were most prevalent.
Conclusions
Ten years after the introduction of higher-valent vaccines, PCV13 serotypes have been reduced among children, but serotypes 3, 19F and 19A persist. Future vaccine formulation would considerably increase serotype coverage.
INVASIVE PNEUMOCOCCAL DISEASE AMONG ADULTS IN GERMANY, TEN YEARS AFTER PCV13 INTRODUCTION (ID 910)
Abstract
Background
Childhood PCV vaccination was generally recommended in Germany in 2006. Apart from a strong direct effect, herd protection effects among non-vaccinated children and adults were observed.
Methods
IPD in adults in Germany has been monitored since 1992. Isolates were serotyped using the Neufeld Quellung reaction.
Results
Prior to introduction of PCVs in infants, 45% of IPD serotypes found among adults were PCV7 types and 70% were PCV13 types. After the start of childhood vaccination, these percentages gradually reduced to 5% and 30% and remained stable in the past five seasons. In 2018-2019, prevalences were of serotypes 3, 4, 19F and 19A were 20.3%, 2.1%, 3.7% and 1.5% respectively. The prevalence of serotype 3 has reached its highest point ever since the introduction of PCVs. Among non-PCV13 types, serotypes 8 (14.0%), 22F (7.2%), 9N (6.4) and 12F (5.0%) were most prevalent. New PCV formulations would cover 38.7% (PCV15) and 65.2% (PCV20).
Conclusions
The herd protection effect of PCVs among adults has reached its limit. No herd protection effect was observed for serotype 3. The data implicate circulation of PCV13 serotypes among adults, which might only be interrupted by direct vaccination.
PNEUMOCOCCAL CARRIAGE AND SEROTYPE DIVERSITY AMONG HEALTHY ADULTS IN BANGLADESH (ID 1095)
Abstract
Background
Streptococcus pneumoniae colonization in the nasopharynx (NP) of elderly reflects transmission in the community and disease risk. Few studies measured pneumococcal carriage among adults. However in developing countries, the data is scare. To address this, we conducted cross-sectional studies during October 2014-February 2015 and July-September 2015. As PCV-10 introduced in Bangladesh on March 2015, both periods are considered baseline data.
Methods
In Mirzapur, a rural area in Bangladesh, 1408 NP swabs were collected from healthy adults (age, 45-95 years) identified during demographic surveillance system household visits. Swabs were cultured for pneumococci and serotyped using quellung reaction.
Results
Pneumococcal colonization rates during the two time periods were 10% (80/762) and 9% (61/646), respectively. Fifty-one different serotypes were detected. Only 13% (19/141) of isolates were PCV-10 serotypes, 22% (31/141) were PCV-13, 33% (46/141) were PCV-20, and 34% (48/141) were 23-valent polysaccharide vaccine serotypes. The most predominant serotypes were 13 (8%), 34 (8%), 3 (6%), 39 (6%), 35B (4%), 18C (4%) and 19F (4%).
Conclusions
Our findings indicate that a diverse set of serotypes cause pneumococcal carriage among older adults in Bangladesh, with 4 of the 7 most predominant not found in existing or forthcoming conjugate vaccine formulations.
ROOM FOR IMPROVEMENT: LOW PNEUMOCOCCAL VACCINATION RATES AND PERSISTENT VACCINE-TYPE DISEASE IN OLDER ADULTS WITH INVASIVE PNEUMOCOCCAL DISEASE IN GERMANY (ID 501)
Abstract
Background
Germany first recommended vaccination with 23-valent polysaccharide vaccine (PPV23) for adults 60 years of age and older in 1998. Despite the longstanding recommendation, pneumococcal vaccination rates among adults are low.
Methods
We are performing a two-year, prospective survey of treating physicians for the lifetime pneumococcal vaccination status of adults ages 60 and older with IPD. Vaccine effectiveness was estimated using the indirect cohort method.
Results
We determined the vaccination status for 839 cases of IPD (of 4751 eligible cases) occurring in older adults in 2018 or 2019. Of these, 616 (73.4%) had received no pneumococcal vaccination prior to illness. 177 (21.1%) cases had been vaccinated with PPV23, 35 (4.2%) had been vaccinated with PCV13, and 11 (1.3%) cases had received both vaccines. 413 cases were caused by PPV23 serotypes; 179 were caused by serotype 3. Preliminary, age-adjusted estimates of vaccine effectiveness for PPV23 are:
| Serotypes | age-adjusted Vaccine Effectiveness | 95% Confidence Intervals | |
| PPV23 serotypes | -20% |
| |
| PPV23 serotypes except 3 | 62% | 45% · 73% | |
| Serotype 3 alone | -128% | -231% · -56% | |
| PPV23nonPCV13 serotypes | 58% | 39% · 71% |
Conclusions
PPV23 provides modest direct protection against IPD caused by most targeted serotypes, but effectiveness against serotype 3 IPD is lacking.
