Kim E. Mulholland, Australia
Murdoch Children's Research Institute Infection & ImmunityPoster Author Of 2 e-Posters
FACTORS ASSOCIATED WITH PNEUMOCOCCAL CARRIAGE IN CHILDREN AND ADULTS IN FIJI, USING FOUR CROSS-SECTIONAL SURVEYS
- Eleanor F. Neal, Australia
- Cattram D. Nguyen, Australia
- Felista T. Ratu, Fiji
- Eileen M. Dunne, United States of America
- Lisi Tikoduadua, Fiji
- Mike Kama, Fiji
- Belinda D. Ortika, Australia
- Laura K. Boelsen, Australia
- Joseph Kado, Australia
- Rachel Devi, Fiji
- Evelyn Tuivaga, Fiji
- Rita C. Reyburn, Australia
- Catherine Satzke, Australia
- Eric Rafai, Fiji
- Kim E. Mulholland, Australia
- Fiona M. Russell, Australia
FIVE YEARS OF PNEUMONIA SURVEILLANCE IN LAO PDR
- Laddaphone Bounvilay, Laos
- Keoudomphone Vilivong,
- Jana Y. Lai, Australia
- Jocelyn Chan,
- Eileen M. Dunne, United States of America
- Kimberly Fox,
- Paul N. Newton, United Kingdom
- Mayfong Mayxay, United Kingdom
- Anonh Xeuatvongsa, Laos
- Kim E. Mulholland, Australia
- Catherine Satzke, Australia
- Audrey Dubot-Pérès, Laos
- David A. Dance, Laos
- Fiona M. Russell, Australia
Author Of 27 Presentations
PNEUMOCOCCAL SEROTYPE 3 IN THE NORTHERN TERRITORY: SEVERE DISEASE DESPITE PNEUMOCOCCAL VACCINATION PROGRAMS (ID 905)
- Heidi Smith-Vaughan, Australia
- Kim M. Hare,
- Anne B. Chang,
- Irani U. Rathnayake,
- Amy V. Jennison,
- Heather Cook,
- Vicki Krause, Australia
- Amanda J. Leach, Australia
- Jemima Beissbarth, Australia
- Robyn L. Marsh,
- Keith Grimwood,
- Helen V. Smith,
- Catherine Satzke, Australia
- Kim E. Mulholland, Australia
- Bart J. Currie,
- Tegan Harris,
HIGH RATES OF MULTIPLE NASOPHARYNGEAL PNEUMOCOCCAL CARRIAGE IN CHILDREN WITH PNEUMONIA IN PAPUA NEW GUINEA FOLLOWING PNEUMOCOCCAL CONJUGATE VACCINE INTRODUCTION (ID 731)
- Rebecca Ford, Papua New Guinea
- Eileen M. Dunne, United States of America
- Jocelyn Chan,
- Lapule Yuasi, Papua New Guinea
- Mition J. Yoannes, Papua New Guinea
- Casey L. Pell, Australia
- Ahmed Alamrousi, Australia
- Jason Hinds, United Kingdom
- Joycelyn J. Sapura, Papua New Guinea
- Birunu Nivio, Papua New Guinea
- Zeena Akunaii, Papua New Guinea
- Kim E. Mulholland, Australia
- Deborah Lehmann, Australia
- William Pomat, Papua New Guinea
- Christopher C. Blyth, Australia
- Catherine Satzke, Australia
- Fiona M. Russell, Australia
Abstract
Background
Pneumococcal carriage rates in Papua New Guinean (PNG) children are among the highest globally. One aim of the multi-site PneuCAPTIVE study is to determine the impact of PCV13 (introduced in 2014) on nasopharyngeal carriage in PNG.
Methods
Nasopharyngeal (NP) swabs and blood were collected from children aged <5 years with moderate or severe pneumonia, and/or suspected meningitis at Eastern Highlands Provincial Hospital or outpatient clinics in Goroka (2016-2018). Pneumococci were identified and quantified by lytA qPCR, and serotyped by microarray. IPD was identified by standard blood culture.
Results
PCV13 coverage was 62%. 1043 were enrolled: 90% had pneumococcal carriage, with median density of 6.59 log10 genome equivalents (GE)/ml (IQR 6.00-7.11). Serotype data were available on 914 cases: 37% were PCV13-types; and 55% had multiple pneumococcal-type carriage. 74 different serotypes and genetic lineages of acapsular pneumococci were identified, the most common being acapsular lineage NT2>19A>15B/C>16F>14. PCV13-type carriage was 28% in vaccinated children vs 46% in unvaccinated children. IPD was confirmed in 7 cases (vaccinated – serotype 1; unvaccinated – serotypes 2, 6B, 15F, 19A, 23A, 29): 4/7 carried the homologous serotype.
Conclusions
There is some evidence of PCV13 being effective against PCV13-types but the high diversity of serotypes in PNG warrants extended valency vaccines.
PNEUMOCOCCAL CARRIAGE AND DENSITY IN MONGOLIAN CHILDREN WITH RADIOLOGICALLY-CONFIRMED PNEUMONIA AND HEALTHY CHILDREN FROM THE COMMUNITY. (ID 387)
- Monica L. Nation, Australia
- Cattram D. Nguyen, Australia
- Eileen M. Dunne, United States of America
- Casey L. Pell, Australia
- Jason Hinds, United Kingdom
- Mukhchuluun Ulziibayar, Mongolia
- Bujinlkham Suuri, Mongolia
- Dashtseren Luvsantseren, Mongolia
- Tuya Mungun, Mongolia
- Kim E. Mulholland, Australia
- Claire Von Mollendorf, Australia
- Catherine Satzke, Australia
PNEUMOCOCCAL CARRIAGE IN CHILDREN WITH PNEUMONIA IN THREE ASIAN COUNTRIES FOLLOWING VACCINE INTRODUCTION (ID 1082)
- Catherine Satzke, Australia
- Eileen M. Dunne, United States of America
- Jocelyn Chan,
- Monica L. Nation, Australia
- Keoudomphone Vilivong, Laos
- Belinda D. Ortika, Australia
- Mimee Laddaphone, Laos
- Rebecca Ford, Papua New Guinea
- Joycelyn J. Sapura, Papua New Guinea
- John Kave, Papua New Guinea
- Cattram D. Nguyen, Australia
- Casey L. Pell, Australia
- Ahmed Alamrousi, Australia
- Jason Hinds, United Kingdom
- Paul N. Newton, United Kingdom
- Anonh Xeuatvongsa, Laos
- B Bunjinlham,
- Christopher C. Blyth, Australia
- David A. Dance, Laos
- William Pomat, Papua New Guinea
- Claire Von Mollendorf, Australia
- Tuya Mungun, Mongolia
- Kim E. Mulholland, Australia
- Fiona M. Russell, Australia
PNEUMOCOCCAL CONJUGATE VACCINE IS EFFECTIVENESS AGAINST HYPOXIC PNEUMONIA IN LAOS, MONGOLIA AND PAPUA NEW GUINEA: A NOVEL CASE-CONTROL VARIANT STUDY (ID 852)
- Fiona M. Russell, Australia
- Cattram D. Nguyen, Australia
- Rupert Weaver, Australia
- Christopher C. Blyth, Australia
- Jocelyn Chan,
- Claire Von Mollendorf, Australia
- Kate Britton, Australia
- David A. Dance, Laos
- Rebecca Ford, Papua New Guinea
- Jana Y. Lai, Australia
- Tuya Mungan, Mongolia
- Paul N. Newton, United Kingdom
- Kim E. Mulholland, Australia
- William Pomat, Papua New Guinea
- Keoudomphone Vilivong, Laos
- Anonh Xeuatvongsa, Laos
Abstract
Background
We describe a novel approach to determine PCV13 effectiveness (VE) against hypoxic pneumonia in children admitted with pneumonia in Lao PDR (Laos), Mongolia and Papua New Guinea (PNG).
Methods
A 3-5 year prospective hospital-based observational study of children <59 months admitted with pneumonia was undertaken. Pneumonia was defined using the 2013 WHO definition. Hypoxia was defined as an oxygen saturation <90% in room air or requiring oxygen supplementation during hospitalisation. PCV13 status was determined by written record. VE was calculated using logistic regression comparing the odds of hypoxia between vaccinated and undervaccinated pneumonia cases. To handle potential confounders a propensity score (PS) analysis using inverse probability of treatment weighting (IPW) was used. In Laos, multiple imputation (MI) analysis was undertaken for missing data.
Results
The VE against hypoxic pneumonia were: in Laos, unadjusted 23% (95% CI: -9, 46%; p=0·14), PS adjusted IPW 37% (6, 57%; p=0·02), MI adjusted 35% (7, 55%; p=0·02); in Mongolia, unadjusted 33% (26, 40%; p<0.001), PS adjusted IPW 33% (16, 47%; p<0.001); and in PNG, unadjusted 6% (-15, 24%; p=0.532), PS adjusted IPW 36% (17, 51%; p=0.001).
Conclusions
Our novel approach shows that PCV13 is effective against hypoxic pneumonia. PCV13 will contribute to reducing child mortality.
INSIGHTS INTO PNEUMOCOCCAL PNEUMONIA USING LUNG ASPIRATES AND NASOPHARYNGEAL SWABS COLLECTED FROM PNEUMONIA PATIENTS IN THE GAMBIA (ID 668)
FIVE YEARS OF PNEUMONIA SURVEILLANCE IN LAO PDR (ID 925)
- Laddaphone Bounvilay, Laos
- Keoudomphone Vilivong,
- Jana Y. Lai, Australia
- Jocelyn Chan,
- Eileen M. Dunne, United States of America
- Kimberly Fox,
- Paul N. Newton, United Kingdom
- Mayfong Mayxay, United Kingdom
- Anonh Xeuatvongsa, Laos
- Kim E. Mulholland, Australia
- Catherine Satzke, Australia
- Audrey Dubot-Pérès, Laos
- David A. Dance, Laos
- Fiona M. Russell, Australia
Abstract
Background
Laos has one of the highest under-five mortality rates in South East Asia, with pneumonia being a leading cause. Hospital-based sentinel site pneumonia surveillance was established at the main tertiary referral hospital in the capital city, Vientiane. We describe the epidemiology of paediatric pneumonia and the detection of potential pathogens from upper respiratory tract samples since PCV13 introduction in 2013.
Methods
From 2013-2019, we enrolled children aged 2-59 months admitted with acute respiratory infection. Oral, throat and nasopharyngeal swabs were taken. Clinical and socioeconomic details were recorded. PCV13 status was recorded from written records. Pneumonia was classified according to the WHO 2013 definition. Multiplex PCR was used to detect respiratory viruses. Pneumococci were detected using lytA qPCR and serotyped using microarray.
Results
1436 were enrolled, of whom 859 had pneumonia. The median age of pneumonia cases were 15 months (IQR 6-21 months), 53.5% had severe pneumonia, 33.5% were hypoxic, and 1.8% died or were discharged unwell. Malnutrition was present in 5.6%. RSV was seasonal and common in young children. PCV13-type carriage declined in vaccinated and under-vaccinated cases.
Conclusions
Childhood pneumonia is a common reason for hospital admission in Laos. There is some evidence of direct and indirect effects of PCV13. RSV is common.
IMMUNOGENICITY OF A SINGLE DOSE OF PCV10 GIVEN AT 18 MONTHS OF AGE AND IMPACT ON NASOPHARYNGEAL CARRIAGE IN VIETNAMESE CHILDREN (ID 735)
- Rachel A. Marimla, Australia
- Beth Temple, Australia
- Thi Trang Dai Vo, Viet Nam
- Thanh V. Phan, Viet Nam
- Trong Toan Nguyen, Viet Nam
- Leena Spry, Australia
- Monica L. Nation, Australia
- Belinda D. Ortika, Australia
- Doan Y. Uyen, Viet Nam
- Cattram D. Nguyen, Australia
- Kathryn Bright, Australia
- Anne Balloch, Australia
- Huu T. Ngoc, Viet Nam
- Kim E. Mulholland, Australia
- Catherine Satzke, Australia
- Paul V. Licciardi, Australia
HEAD TO HEAD COMPARISONS AT 2, 4, AND 7 MONTHS, FOLLOWING STANDARD AND COMBINED PHID-CV10 AND PCV13 SCHEDULES. (ID 429)
- Amanda J. Leach, Australia
- Nicole Wilson, Australia
- Jemima Beissbarth, Australia
- Kim E. Mulholland, Australia
- Mathuram Santosham, United States of America
- Peter McIntyre, Australia
- Paul V. Licciardi, Australia
- Mark Chatfield, Australia
- Victor Oguoma, Australia
- Jonathan Carapetis, Australia
- Sue Skull, Australia
- Heidi Smith-Vaughan, Australia
- Vicki Krause, Australia
- Ross Andrews, Australia
- Peter Morris, Australia
- Paul Torzillo, Australia
Abstract
Background
Australian Aboriginal children are at high risk of early infection withStreptococcus pneumoniae and non-typeable Haemophilus influenzae (NTHi). We evaluated immunogenicity against 10 shared serotypes of a 4-dose combination schedule of PHiD-CV10 at 1-2-4 months plus PCV13 at 6 months, compared with standard 2-4-6 month schedules.
Methods
Infants were allocated (1:1:1) at 28 to 38 days of age, to 3-dose schedules of PCV13 (P) or PHiD-CV10 (S) at 2-4-6 months (_PPP or _SSS), or a combination schedule at 1-2-4-6 months (SSSP). Immunogenicity was measured at 2, 4, and 7 months.
Results
At 2 months the SSSP combination was superior to pre-vaccination (VTs other than 6B, 19F, or 23F). At 4 months SSSP was superior to _PPP (9 VTs) and _SSS (7 VTs), and _SSS was superior to _PPP (8 VTs). At 7 months, SSSP was superior to _PPP (1, 6B, 9V, 19F and 23F) and _SSS (8 VTs), and _PPP was superior to _SSS (8 VTs). OPA supports the SSSP schedule, particularly against 1, 6B, and 23F.
Conclusions
The 1-2-4-6 month schedule (SSSP) was superior at 2, 4, and 7 months of age compared to _SSS or _PPP, particularly for 1, 6B, and 23F at 7 months. At 4 months, _SSS was superior to _PPP.
STUDY PARTICIPANT WITHDRAWALS: LESSONS LEARNT FROM A VACCINE TRIAL IN INFANTS IN HO CHI MINH CITY, VIETNAM (ID 1105)
Abstract
Background
Subject recruitment and retention are crucial factors contributing to the success of clinical trials. We describe the experience from a clinical trial of
pneumococcal conjugate vaccines currently underway in Ho Chi Minh City.
Methods
Subject recruitment and retention are crucial factors contributing to the success of clinical trials. We describe the experience from a clinical trial of
pneumococcal conjugate vaccines currently underway in Ho Chi Minh City.
Results
2,501 subjects were recruited at 2 months of age. To date 1,612 subjects have completed their final 24 month visit. The last 24 month visit is due in May 2020. We have a current withdrawal rate of 7.8%. The most common reason for withdrawal is moving outside the study area. Withdrawal rates and reasons by study group will be presented at the conference
Conclusions
Recruiting subjects who intend to remain in the study area is a challenge for clinical trials involving follow up of children over a number of years. Involving staff from subjects' local health clinics helps to keep the withdrawal rate down.
SUBSTANTIAL INDIRECT PROTECTION AGAINST IPD AND PNEUMONIA HOSPITALISATIONS AT LOW LEVELS OF VACCINE COVERAGE IN AUSTRALIA, YET HIGH COVERAGE REQUIRED FOR NEAR-ELIMINATION (ID 854)
COMMUNICATING PCV13 IMPACT RESULTS IN LAO PDR, USING A MULTIMEDIA APPROACH (ID 690)
- Shereen Labib, Australia
- Jocelyn Chan,
- Keoudomphone Vilivong, Laos
- Mimee Laddaphone, Laos
- Jana Y. Lai, Australia
- Lauren Franzel-Sassanpour, Laos
- Eileen M. Dunne, United States of America
- Paul N. Newton, United Kingdom
- Kim E. Mulholland, Australia
- Audrey Dubot-Pérès, Laos
- Catherine Satzke, Australia
- Molina Choummanivong, Laos
- Vanphanom Sychareun, Laos
- Anonh Xeuatvongsa, Laos
- Mayfong Mayxay, United Kingdom
- David A. Dance, Laos
- Fiona M. Russell, Australia
Abstract
Background
In 2013, Lao PDR introduced PCV13 with Gavi support. WHO requested a PCV13 impact evaluation as the Ministry of Health required evidence of PCV13 impact. Our project included a variety of community and hospital-based carriage and disease studies.
Methods
We partnered with Lao Ministry of Health and WHO, the key end-users, from the outset. We performed high quality research by collaborating with established international research institutions in Laos, the Lao Oxford Mahosot Hospital-Wellcome Trust Research Unit (LOMWRU) and the leading Lao medical university, The University of Health Sciences, to undertake the research. We developed an infographic and a video of the results.
Results
We disseminated our results to immunisation policy makers at the Lao Ministry of Health, WHO (Laos office and Geneva) and our funders, Gavi, the Vaccine Alliance, and the Bill & Melinda Gates Foundation. Our results were presented to the Lao paediatricians and NITAG members; and at various local, regional and international conferences. The Laos Minister of Health presented the findings to the Gavi Board. The video and infographic were launched on social media and hosted on our institutions’ (MCRI and University of Melbourne) webpage, to coincide with World Pneumonia Day.
Conclusions
This multipronged approach ensured wide dissemination of findings.
INCREASED PCV10 IMMUNOGENICITY FOLLOWING A TWO-DOSE PRIMARY SERIES SEPARATED BY 4 MONTHS COMPARED WITH 2 MONTHS IN VIETNAMESE INFANTS (ID 963)
- Paul V. Licciardi, Australia
- Beth Temple, Australia
- Dai V. Trang, Australia
- Trong Toan Nguyen, Viet Nam
- Uyen Y. Doan, Viet Nam
- Cattram D. Nguyen, Australia
- Thanh V. Phan, Viet Nam
- Kathryn Bright, Australia
- Rachel A. Marimla, Australia
- Anne Balloch, Australia
- Tran Ngoc Huu, Viet Nam
- Kim E. Mulholland, Australia
DISEASE FEATURES OF VIETNAMMESE INFANTS FROM HOSPITAL ADMISSIONS OF A PNEUMOCOCCAL VACCINE PHASE 3 CLINICAL TRIAL (ID 741)
CLINICAL CHARACTERISTICS OF ADULT PNEUMONIA CASES IN THE ERA OF CHILDHOOD PCV13 VACCINATION IN MONGOLIA, 2015-2018 (ID 502)
NASOPHARYNGEAL PNEUMOCOCCAL DENSITY IS ASSOCIATED WITH SEVERE PNEUMONIA IN YOUNG CHILDREN IN LAO PDR (ID 856)
- Olivia J. Carr, Australia
- Keoudomphone Vilivong, Laos
- Mimee Laddaphone, Laos
- Eileen M. Dunne, United States of America
- Jana Y. Lai, Australia
- Jocelyn Chan,
- Malisa Vongsakid, Laos
- Chanthaphone Siladeth, Laos
- Belinda D. Ortika, Australia
- Mayfong Mayxay, United Kingdom
- Paul N. Newton, United Kingdom
- Lien Anh Ha H. Do, Australia
- Kim E. Mulholland, Australia
- Audrey Dubot-Pérès, Laos
- Catherine Satzke, Australia
- David A. Dance, Laos
- Fiona M. Russell, Australia
Abstract
Background
Pneumococcal nasopharyngeal colonisation density >6.9 log10 copies/mL is associated with primary endpoint pneumonia, very severe pneumonia and hypoxic pneumonia. Few studies have explored the association between pneumococcal density and severe pneumonia. We determined the association between nasopharyngeal pneumococcal density and children with severe pneumonia in Laos.
Methods
A prospective observational study was conducted at Mahosot Hospital. Children <5 years of age admitted with ARI were recruited (2014 to mid-2018). Clinical and demographic data were collected alongside with nasopharyngeal swabs. Severe pneumonia was classified according to the WHO 2013 definition. Pneumococci were detected and quantified by lytA qPCR. A logistic regression model deterimined the association between pneumococcal density and severe pneumonia, after adjusting for potential confounders.
Results
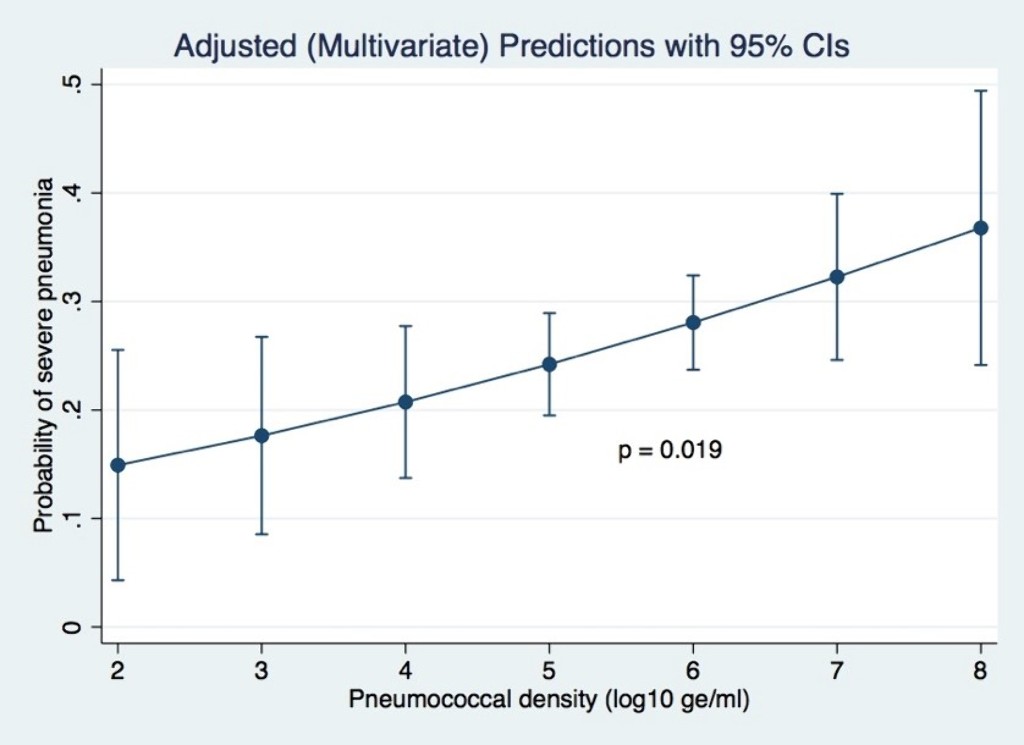
Of 1,289 participants enrolled, 32.2% had severe pneumonia. After adjusting for potential confounders (age, ethnicity, residential location, living with children <5 years, exposure to cigarette smoke, monthly income, PCV13 vaccination status and co-detection of RSV), pneumococcal density was positively associated with severe pneumonia (adjusted odds ratio 1.4; 95% CI 1.1–1.8; p=0.019).
Conclusions
Pneumococcal carriage density is associated with the probability of severe pneumonia in children in this setting.
IMPACT OF A SINGLE DOSE OF PCV10 OR PCV13 ON NASOPHARYNGEAL PNEUMOCOCCAL CARRIAGE IN VIETNAMESE CHILDREN DURING THE FIRST YEAR OF LIFE (ID 696)
- Hoan Thi Pham, Viet Nam
- Beth Temple, Australia
- Thi Trang Dai Vo, Viet Nam
- Thanh V. Phan, Viet Nam
- Loc Thuy Ho Nguyen, Viet Nam
- Anh H.V Nguyen,
- Belinda D. Ortika, Australia
- Kathryn Bright, Australia
- Catherine Satzke, Australia
- Nevio D. Sarmento, Timor-Leste
- Jemima Beissbarth, Australia
- Heidi Smith-Vaughan, Australia
- Thuong V. Nguyen, Viet Nam
- Kim E. Mulholland, Australia
Abstract
Background
Reduced-dose schedules of pneumococcal conjugate vaccine (PCV) could increase the accessibility and use of PCV in low and middle-income countries.
Methods
Groups within the Vietnam Pneumococcal Trial II receive PCV10 and PCV13 in a 1+1 schedule at 2 and 12 months of age, or no vaccine. Nasopharyngeal swabs were collected at 6 and 12 months of age to show the impact of the 2-month dose on pneumococcal carriage.
Results
Based on analysis to date of 1152 of 3200 swabs, vaccine-type carriage was low. In unvaccinated participants, PCV10 and PCV13-type carriage were 5.1% and 10.4% at 6 months, and 8.3% and 12.0% at 12 months, respectively. A dose of PCV10 transiently reduced vaccine-type carriage at 6 months of age (3/178 [1.7%] versus 18/355 [5.1%]).
Conclusions
With the exception of the PCV10 group at 6 months of age, both vaccine-type and non-vaccine-type carriage rates were similar among PCV10-vaccinated participants, PCV13-vaccinated participants and unvaccinated controls at 6 and 12 months of age. Based on preliminary data, a single dose of PCV at 2 months of age does not appear to reduce pneumococcal carriage during the first year of life in this population.
INTERCHANGEABILITY OF PHID-CV10 AND PCV13 IN PRIMARY COURSE SCHEDULES (ID 204)
- Amanda J. Leach, Australia
- Nicole Wilson, Australia
- Jemima Beissbarth, Australia
- Kim E. Mulholland, Australia
- Mathuram Santosham, United States of America
- Peter McIntyre, Australia
- Paul V. Licciardi, Australia
- Mark Chatfiled, Australia
- Victor Oguoma, Australia
- Jonathan Carapetis, Australia
- Sue Skull, Australia
- Heidi Smith-Vaughan, Australia
- Vicki Krause, Australia
- Ross Andrews, Australia
- Peter Morris, Australia
- Paul Torzillo, Australia
Abstract
Background
In remote communities of northern Australia, we previously demonstrated that the onset of otitis media (OM) in Aboriginal infants was preceded by acquisition of bacterial pathogens that colonise the nasopharynx (NP) soon after birth. We aimed to determine safety and effectiveness of mixed vaccine schedules against early infection due to non-typeable Haemophilus influenzae and Streptococcus pneumoniae.
Methods
In an open-label controlled trial, we randomised (1:1:1) Aboriginal infants at 28 to 38 days of age, to either Prevenar13™ (P, PCV13) at 2-4-6 months of age (_PPP), Synflorix™ (S, PHiD-CV10) at 2-4-6 months (_SSS), or Synflorix at 1-2-4 months plus Prevenar13 at 6 months (SSSP). Primary outcomes (assessor-blinded) were immunogenicity at 7 months of age against pneumococcal serotypes 3, 6A, and 19A, and protein D (GMCs and proportions of infants with IgG > 0·35 µg/mL or > 100 EL.U/mL, respectively). Secondary immunogenicity outcomes at 2 and 4 months are also reported.
Results
A 4-dose early 1-2-4-6 month combination schedule of Synflorix plus Prevenar13 (SSSP) provided superior overall immune protection against serotypes 3, 6A, 19A, and protein D, compared to standard 3-dose 2-4-6 month schedules (_SSS or _PPP).
Conclusions
These vaccines can be combined safely and effectively within this primary schedule, with no evidence of immune suppression.
MONITORING PCV13 IMPACT USING NASOPHARYNGEAL CARRIAGE SURVEILLANCE AMONG CHILDREN WITH PNEUMONIA IN MONGOLIA (ID 965)
- Purevsuren Batsaikhan, Mongolia
- Jocelyn Chan,
- Tuya Mungun, Mongolia
- Eileen M. Dunne, United States of America
- Sophie La Vincente, Australia
- Dorj Narangerel, Mongolia
- Monica L. Nation, Australia
- J Hinds,
- Cattram D. Nguyen, Australia
- Mukhchuluun Ulziibayar, Mongolia
- Catherine Satzke, Australia
- Kim E. Mulholland, Australia
- Claire Von Mollendorf, Australia
- Fiona M. Russell, Australia
Abstract
Background
In 2015, Mongolia was among the earliest countries in Asia to introduce PCV. To monitor the impact of PCV13 introduction, we commenced nasopharyngeal carriage surveillance among children with pneumonia 6 months prior to vaccine introduction.
Methods
We recruited children 2-59 months of age presenting with pneumonia to district hospitals and the national Maternal and Child Health hospital in two districts in Ulaanbaatar. Clinical and demographic data, vaccination status and nasopharyngeal swabs were collected. A random sample of swabs were selected for testing each month. Samples were examined by lytA qPCR, with positives serotyped by microarray.
Results
We recruited 4980 children and tested 983 children from November 2015 to April 2018. The median age was 1.27 and 25.81% of cases were vaccinated in the first and second year following PCV13 introduction, respectively. 474 and 48.22% had received antibiotics in the 48 hours before admission.
Conclusions
Following PCV13 introduction in Mongolia, the prevalence of pneumococcal carriage remained stable while the prevalence of PCV13-type carriage decreased among children with pneumonia. Reductions in PCV13 carriage likely correspond to reductions in disease due to PCV13 types, since carriage is a precursor for disease.
A DYNAMIC MODEL TO DETERMINE FACTORS REQUIRED FOR ELIMINATION OF VACCINE-TYPE CARRIAGE FOLLOWING PNEUMOCOCCAL CONJUGATE VACCINE INTRODUCTION IN THE ASIA-PACIFIC (ID 850)
- Jocelyn Chan,
- Virginia Pitzer, United States of America
- Cattram D. Nguyen, Australia
- Eileen M. Dunne, United States of America
- Christopher C. Blyth, Australia
- David A. Dance, Laos
- Rebecca Ford, Papua New Guinea
- Jana Y. Lai, Australia
- Sophie La Vincente, Australia
- Deborah Lehmann, Australia
- Siddhartha S. Datta, Laos
- Kimberley Fox, Philippines
- Monica L. Nation, Australia
- Jason Hinds, United Kingdom
- Tuya Mungun, Mongolia
- Paul N. Newton, United Kingdom
- William Pomat, Papua New Guinea
- Keoudomphone Vilivong, Laos
- Claire Von Mollendorf, Australia
- Anonh Xeuatvongsa, Laos
- Catherine Satzke, Australia
- Kim E. Mulholland, Australia
- Daniel M. Weinberger, United States of America
- Fiona M. Russell, Australia
THE IMPACT OF THE 10-VALENT PNEUMOCOCCAL CONJUGATE VACCINE ON INVASIVE DISEASE IN FIJI: A RETROSPECTIVE REVIEW (ID 504)
- Felista T. Ratu, Fiji
- Rita C. Reyburn, Australia
- Evelyn Tuivaga, Fiji
- Eileen M. Dunne, United States of America
- Devina Nand, Fiji
- Joseph Kado, Australia
- Lisi Tikoduadua, Fiji
- Kimberley Fox, Philippines
- Rachel Devi, Fiji
- Catherine Satzke, Australia
- Susan Ballard, Australia
- Eric Rafai, Fiji
- Kim E. Mulholland, Australia
- Stefan Flasche, United Kingdom
- Mike Kama, Fiji
- Fiona M. Russell, Australia
PROGRESS IN THE RANDOMISED CONTROLLED TRIAL "TRIAL OF SIMPLIFIED PNEUMOCOCCAL VACCINATION IN VIETNAM II: THE HERD IMMUNITY APPROACH". (ID 864)
CO-ADMINISTRATION OF 10-VALENT PNEUMOCOCCAL CONJUGATE VACCINE AND MEASLES VACCINE DOES NOT IMPACT ON THE IMMUNOGENICITY OF MEASLES VACCINE (ID 727)
FACTORS ASSOCIATED WITH PNEUMOCOCCAL CARRIAGE IN CHILDREN AND ADULTS IN FIJI, USING FOUR CROSS-SECTIONAL SURVEYS (ID 224)
- Eleanor F. Neal, Australia
- Cattram D. Nguyen, Australia
- Felista T. Ratu, Fiji
- Eileen M. Dunne, United States of America
- Lisi Tikoduadua, Fiji
- Mike Kama, Fiji
- Belinda D. Ortika, Australia
- Laura K. Boelsen, Australia
- Joseph Kado, Australia
- Rachel Devi, Fiji
- Evelyn Tuivaga, Fiji
- Rita C. Reyburn, Australia
- Catherine Satzke, Australia
- Eric Rafai, Fiji
- Kim E. Mulholland, Australia
- Fiona M. Russell, Australia
Abstract
Background
We describe factors associated with pneumococcal nasopharyngeal carriage in Fiji, using data from annual (2012-2015) cross-sectional surveys, pre- and post-introduction of ten-valent pneumococcal conjugate vaccine (PCV10).
Methods
Infants (5-8 weeks), toddlers (12-23 months), children (2-6 years), and their caregivers participated. Pneumococci were detected using lytA qPCR, with molecular serotyping by microarray. We used logistic regression to determine predictors of pneumococcal carriage.
Results
There were 8,109 participants. Pneumococcal carriage was associated with: years post-PCV10 introduction (global P<0.001), indigenous iTaukei ethnicity (aOR 2.74 [95% CI 2.17-3.45] P<0.001); young age (global P<0.001); urban residence (aOR 1.45 [95% CI 1.30-2.57] P<0.001); living with >2 children <5 years (aOR 1.42 [95% CI 1.27-1.59] P<0.001); poverty (aOR 1.44 [95% CI 1.28-1.62] P<0.001); and upper respiratory tract infection symptoms (aOR 1.77 [95% CI 1.57-2.01] P<0.001). Factors associated with PCV10 and non-PCV10 carriage were similar to those associated with overall carriage. Additionally, PCV10 carriage was associated with PCV10 vaccination (0.58 [95% CI 0.41-0.82] P=0.002) and cigarette smoke exposure (aOR 1.21 [95% CI 1.02-1.43] P=0.031, while non-PCV10 carriage was not associated with years post-PCV10 introduction.
Conclusions
Introduction of PCV10 reduced the odds of overall and PCV10 pneumococcal carriage in Fiji. Indigenous iTaukei ethnicity was positively associated with carriage after adjustment for PCV10.
IMMUNOGENICITY OF A SINGLE DOSE OF PCV10 OR PCV13 AT 2 MONTHS OF AGE IN VIETNAM (ID 1007)
Abstract
Background
A 1+1 pneumococcal conjugate vaccine (PCV) schedule would increase its affordability for low and middle-income countries. The Vietnam Pneumococcal Trial II includes infants randomised to receive a 1+1 schedule of PCV10 or PCV13 at 2 and 12 months of age. This study measured the immunogenicity of a single dose of either PCV given at 2 months of age.
Methods
Serotype-specific IgG was measured at 3 and 12 months of age in PCV-vaccinated and unvaccinated controls using a WHO ELISA method.
Results
At 3 months, both vaccinated groups had higher antibody levels than controls for 7/10 serotypes (all except 6B, 14, 23F). The PCV10 group had higher antibody levels than the PCV13 group for 5/10 serotypes. Similar results were seen at 12 months, with higher antibody levels for all serotypes in the PCV10 group and 8/10 serotypes (all except 6V, 23F) in the PCV13 group compared with controls and higher antibody levels in the PCV10 than the PCV13 group for 7/10 serotypes.
Conclusions
A single dose of PCV in infancy is immunogenic and likely to provide some direct protection until the time of the booster dose. There may be a difference in the degree of protection offered by different PCVs.
DETERMINING THE PNEUMOCOCCAL CONJUGATE VACCINE COVERAGE REQUIRED FOR INDIRECT PROTECTION IN LAOS, MONGOLIA AND PAPUA NEW GUINEA (ID 851)
- Jocelyn Chan,
- Cattram D. Nguyen, Australia
- Eileen M. Dunne, United States of America
- Christopher C. Blyth, Australia
- David A. Dance, Laos
- Rebecca Ford, Papua New Guinea
- Jana Y. Lai, Australia
- Sophie La Vincente, Australia
- Deborah Lehmann, Australia
- Siddhartha S. Datta, Laos
- Kimberley Fox, Philippines
- Monica L. Nation, Australia
- Jason Hinds, United Kingdom
- Tuya Mungun, Mongolia
- Paul N. Newton, United Kingdom
- William Pomat, Papua New Guinea
- Keoudomphone Vilivong, Laos
- Claire Von Mollendorf, Australia
- Anonh Xeuatvongsa, Laos
- Catherine Satzke, Australia
- Kim E. Mulholland, Australia
- Fiona M. Russell, Australia
COMPARISON OF IMMUNOGENICITY OF TEN- AND THIRTEEN-VALENT PNEUMOCOCCAL CONJUGATE VACCINES – A SYSTEMATIC REVIEW AND NETWORK META-ANALYSIS (ID 567)
- Shuo Feng, United Kingdom
- Julie McLellan, United Kingdom
- Nicola Pidduck, United Kingdom
- Julian Higgins, United Kingdom
- Maria D. Knoll, United States of America
- Mark Jit, United Kingdom
- Yoon Hong Choi, United Kingdom
- Beth Temple, Australia
- Kim E. Mulholland, Australia
- Andrew J. Pollard, United Kingdom
- Merryn Voysey, United Kingdom
Abstract
Background
Streptococcus pneumoniae causes substantial morbidity and mortality globally, with serotype-specific disease burden varying geographically. Currently there are two widely used pneumococcal conjugate vaccines (PCV). Evidence is limited regarding their comparative serotype-specific immunogenicity and efficacy.
Methods
We conducted a systematic-review and network meta-analysis of studies in which the immunogenicity of PCVs was directly compared in head-to-head randomised trials. Network meta-analysis incorporates both direct pair-wise comparisons and indirect comparisons (e.g. PCV10 vs PCV7, and PCV13 vs PCV7) thereby increasing overall statistical precision for the main comparison of interest (PCV10 vs PCV13). The difference in immunogenicity, as measured by geometric mean ratio (GMR), was examined by serotype.
Results
27 trials were included in the network meta-analysis, 4 directly comparing PCV10 and PCV13, 7 comparing PCV7 and PCV10, and 16 comparing PCV7 and PCV13. For the 10 common serotypes, immunogenicity at 1 month after the primary vaccination series was higher for PCV13 for 7 serotypes, with GMRs ranging from 1.18 to 2.50. In contrast, serotype 5, 18C and 19F favoured PCV10. Similar results were observed post-booster dose except for serotype 5 and 7F.
Conclusions
Variation in serotype-specific immunogenicity exists between PCV10 and PCV13, indicating that further investigation into whether this translates to heterogeneity in protection is needed.

