850 Presentations
SIMPLIFYING THE MEAL-TIME BOLUS CALCULATION: ACCURACY OF A PERSONALIZED SMALL/MEDIUM/LARGE (S/M/L) OPTION
Abstract
Background and Aims
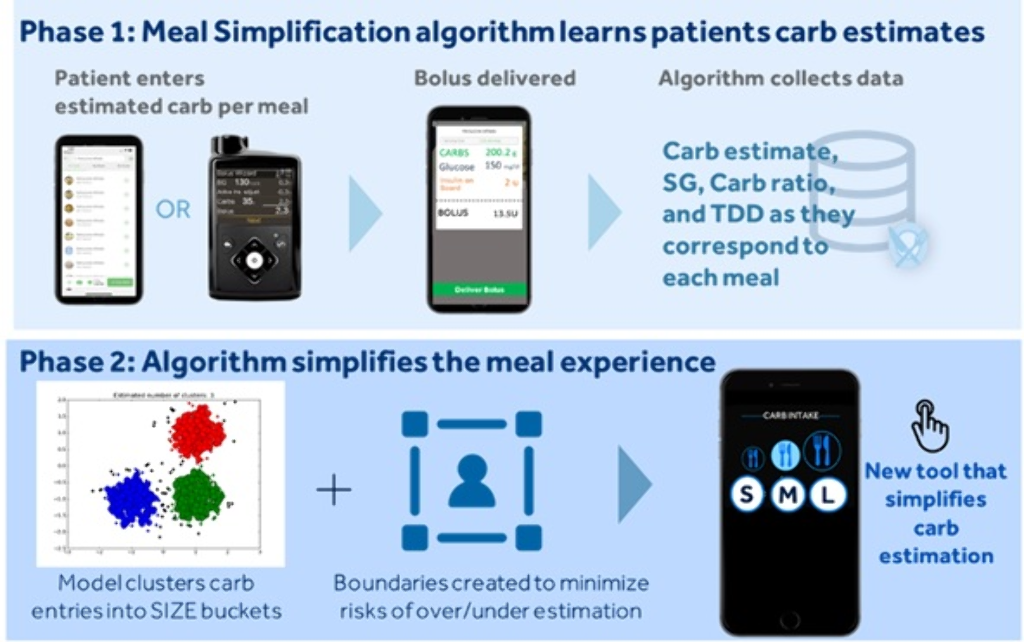
To simplify the complexity of carb counting, a Meal Simplification (MS) algorithm was developed that allows one to choose a user-specific small, medium, or large carb-entry size (S/M/L), instead of entering estimated carbs to the nearest gram.
Methods
The MS system works in two phases (Figure). Phase-1 is the learning phase where traditional carb-counting and entry are carried out via insulin pump or app. Once enough data are collected and uploaded to the cloud, the system enters Phase-2 where it uses machine-learning techniques to identify clusters of meals based on meal size. A set of common S/M/L meal sizes with a corresponding range of carb values and a centroid carb value, on which a meal bolus amount is calculated, is determined. The user has the option to override the centroid carb value to adjust for the actual size of a specific meal. The algorithm updates the S/M/L carb-entry sizes to accommodate for these changes. An in-silico experiment was conducted with 2087 virtual Type 1 Diabetes subjects using the Medtronic Advanced Hybrid Closed-loop system.
Results
For mealtime boluses, the Control group involved the traditional carb-counting method. The Intervention group involved personalized S/M/L meal-clustering. Time spent between 70-180 mg/dL, <70 mg/dL, >180 mg/dL, and >250 mg/dL for the Control versus Intervention arm were 85.1±6.8% versus 84.5±6.6%, 2.0±2.6% versus 2.1±2.5%, 12.9±7.1% versus 13.4±7.1%, and 2.1±2.1% versus 2.3±2.6%, respectively.
Conclusions
Personalized Meal Simplification algorithms for pump users are expected to reduce the burden of carb counting to the nearest gram, without increasing hypoglycemia/hyperglycemia exposure.
COST EFFECTIVENESS ANALYSIS OF MINIMEDTM 670G SYSTEM VERSUS CONTINUOUS SUBCUTANEOUS INSULIN INFUSION, IN INDIVIDUALS WITH TYPE1 DIABETES IN THE UNITED KINGDOM
Abstract
Background and Aims
This study assesses the long-term cost-effectiveness of the MiniMedTM 670G hybrid closed loop system versus Continuous Subcutaneous Insulin Infusion (CSII) alone in people with type 1 diabetes (T1D) in UK.
Methods
The IQVIA-CORE-Diabetes-Model was used to perform cost-effectiveness analysis over patient lifetime. Clinical data were sourced from pivotal clinical study comparing MiniMedTM 670G system with CSII in people with T1D; in which the use of MiniMedTM 670G system was associated with a reduction in HbA1c of 0.5% (5.5 mmol/mol), from 7.4% (57 mmol/mol) at baseline to 6.9% (52 mmol/mol) at the end of the study. Cost data, expressed in 2018 British pounds (GBP), were obtained from UK reference-prices and the published literature.
Results
The MiniMedTM 670G system was associated with a quality-adjusted life-year (QALY) gain of 1.74 but higher overall costs versus CSII, leading to an incremental cost-effectiveness ratio (ICER) of GBP 20,421 per QALY gained. Use of MiniMedTM 670G system resulted in a lower cumulative incidence of diabetes-related complications. Higher MiniMedTM 670G system acquisition costs were partially offset by reduced complication costs. In patients with HbA1c≥7.5% at baseline, MiniMedTM 670G system was associated with 2.02 incremental QALYs versus CSII, yielding an ICER of GBP12,892 per QALY gained. Extensive sensitivity analysis on key drivers confirmed the robustness of results.
Conclusions
The MiniMedTM 670G system was associated with clinical benefits and quality of life improvements in people with T1D relative to CSII. At a willingness-to-pay threshold of GBP20,000 per QALY-gained, MiniMedTM 670G System likely represents a cost-effective treatment option for people with T1D in UK.
EFFECTIVENESS AND SAFETY OF A HYBRID CLOSED LOOP SYSTEM IN CHILDREN AND ADOLESCENTS WITH TYPE 1 DIABETES: 1-YEAR EVALUATION.
Abstract
Background and Aims
We evaluated the efficacy and safety of a hybrid closed loop (HCL) system after its arrive in Italian market in October 2018 and during 1-year follow-up.
Methods
We prospectively analyzed data of all patients who started the Minimed 670G system, (Medtronic, CA, USA) after 1 year since its arrival. Main outcome is the time in range (TIR). Secondary outcomes are HbA1c change from baseline, time in hypo (70-54 and <54), time in hyper (>180 mg/dl and >250), coefficient of variation (CV).
Results
After 1-year follow-up, 52 patients (mean age 11±5 yr s, range 6-20 yrs, diabetes duration 7±4 yrs) had a TIR of 71.3±17.6% when considering 70-180 mg/dl (range 32-97%), using the system in automatic mode for 84% of time. HbA1c significantly improved (7.12±0.87 vs 7.62±1.7, p=0.001). Time in hypo, in hyper and CV were respectively 1.8±1.9%, 0.63±1.0%, 18.2±8.0, 8.0 ±11.3 and 33±5.7% (n.v. <36%). A significant correlation has been observed between TIR and time in automatic mode (r=0.891, p=0.001). Considering the patients using the system >70% in automatic mode (n=42, 81%), TIR was 80.9±7.6%, using the system in automatic mode for 93% of time. Time in hypo, in hyper and CV were respectively 2±2.1%, 0.7±1.1%, 14.3±5.7, 2.1 ±1.4 and 31±5 %
Conclusions
670G system is effective to achieve a high TIR. A systematic educational pathway, as the one used by 640G users, could help reach recommended target even in pediatric population, overcoming some constrains observed using 670G system. A decalogue to help users will be proposed.
CONTROL AND ESTIMATION OF DIABETES MELLITUS BY ROBUST FIXED POINT TRANSFORMATION METHOD
PERFORMANCE OF OMNIPOD PERSONALIZED MODEL PREDICTIVE CONTROL ALGORITHM WITH MULTIPLE SETPOINTS AND MEAL AND EXERCISE CHALLENGES IN CHILDREN AGED 2-12 YEARS WITH TYPE 1 DIABETES
- Jennifer Sherr, United States of America
- Bruce A. Buckingham, United States of America
- Gregory P. Forlenza, United States of America
- Alfonso Galderisi, United States of America
- Laya Ekhlaspour, United States of America
- R. Paul Wadwa, United States of America
- Melinda Zgorski, United States of America
- Ryan Kingman, United States of America
- Cari Berget, United States of America
- Joon Bok Lee, United States of America
- Jason O’connor, United States of America
- Bonnie Dumais, United States of America
- Todd Vienneau, United States of America
- Lauren Huyett, United States of America
- Trang Ly, United States of America
Abstract
Background and Aims
The Omnipod hybrid closed-loop (HCL) personalized model predictive control (MPC) algorithm was assessed in children aged 2-12y with type 1 diabetes (T1D) using an investigational device. This study provided the first evaluation at multiple glucose setpoints and with missed meal boluses in this age group.
Methods
Participants aged 2-12y with T1D and A1C<10.0% using CSII or MDI were eligible for a 72-96-h HCL study conducted in a supervised free-living hotel setting. At HCL start, the glucose setpoint was 150mg/dL, and at 24-48h it was lowered to 120mg/dL. The system was stress-tested with 1-2 missed lunch boluses, high fat dinners, and daily moderate-intensity exercise. Endpoints were mean glucose and percentage time <54, <70, 70-180, >180, and ≥250mg/dL at each setpoint.
Results
Participants (n=9) were (mean±SD): age 7.1±2.2y, T1D duration 3.0±1.6y, and A1C 7.6±1.0%. Glycemic outcomes at each setpoint are reported in the Table. Despite stress-testing the system with missed meal bolus challenges (20-75g carbohydrate) and exercise, percentage time in target range (TIR) from 70-180 mg/dL was ~65% regardless of the system setpoint. Mean glucose was 158±19mg/dL and 165±13mg/dL at the 120mg/dL and 150mg/dL setpoints, respectively.
Conclusions
The Omnipod personalized MPC algorithm performed well and was safe for up to 5 days of use in children aged 2-12y with T1D when stress-tested at 2 different setpoints under challenging conditions. With recent consensus guidelines recommending TIR >60% for youth, our data demonstrate the feasibility of attaining this goal despite the challenges imposed in this trial.

NIGHT TIME GLYCEMIC CONTROL BEFORE AND AFTER MINIMED™ 670G SYSTEM USE BY CHILDREN WITH T1D 2-6 YEARS OF AGE
Abstract
Background and Aims
Adults who experienced increased overnight glucose variability or insulin delivery needs (akin to dawn phenomenon) during the MiniMed™ 670G system trial baseline run-in, exhibited improved overnight glycemic control after three months of Auto Mode use.1 The impact of Auto Mode use on the overnight glycemia of the youngest pediatric cohort of the MiniMed™ 670G system trials was investigated.
Methods
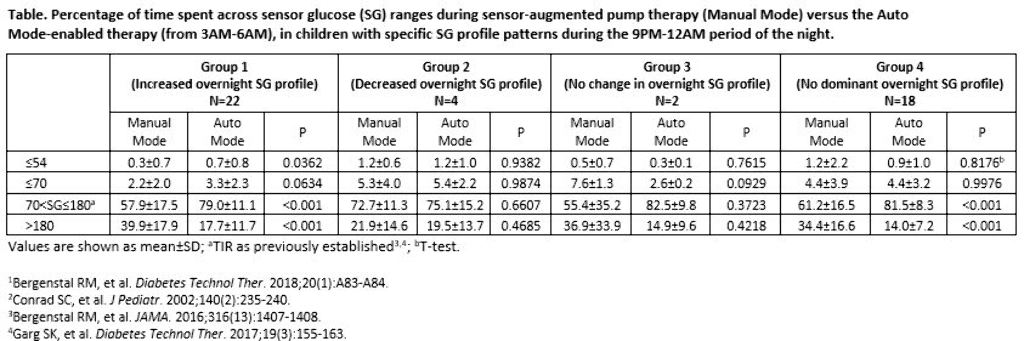
The 9PM-12AM sensor glucose (SG) profile of participants aged 2-6 years with T1D was analyzed, based on Conrad et al. 2002.2 Profiles were categorized as displaying a 1) >10 mg/dL increase in SG, 2) >10 mg/dL decrease in SG, or 3) No change in SG (≤10 mg/dL decrease or increase) and for >50% of the two-week baseline. If an SG profile did not present with an aforementioned pattern, it was categorized into a fourth group (No dominant SG profile). The percentage of early morning (3AM-6AM) time spent across SG levels were compared.
Results
There were 22, 4, 2, and 18 participants in Groups 1, 2, 3, and 4, respectively, during baseline. After three months of Auto Mode use, participants numbered 11, 1, 1, and 33, respectively. Significantly improved time in target glucose range (TIR, >70-180 mg/dL) was observed in Groups 1 and 4. Time spent at ≤70 mg/dL increased for Group 1, but was not significant.
Conclusions
Children with T1D displayed different profiles of overnight SG variability during the MiniMed™ 670G system trial. Regardless of the varied profile patterns observed, the MiniMed™ 670G system safely and effectively improved or did not change overnight TIR.
DEVELOPMENT PLATFORM FOR ARTIFICIAL PANCREAS ALGORITHMS
Abstract
Background and Aims
Assessing algorithms of artificial pancreas systems is critical in developing automated and fault-tolerant solutions that work outside clinical settings. The development and evaluation of algorithms can be facilitated with a platform that conducts virtual clinical trials. We present a clinically validated cloud-based distributed platform that supports the development and comprehensive testing of single and dual-hormone algorithms for type 1 diabetes.
Methods
The platform is built on principles of object-oriented design, and runs user algorithms in real-time virtual clinical trials utilizing a multi-threaded environment enabled by concurrent execution over a cloud infrastructure. Users import a plugin into their algorithms (Matlab, Python, or Java) to connect to the platform. Once connected, users interact with a graphical interface to design experimental protocols for their trials. Protocols include trial duration in days, meal times and amounts, variability in meal times and amounts, carbohydrate counting errors, snacks, and onboard insulin levels. We validate and integrate into the platform a glucoregulatory system of ordinary differential equations (ODEs) parameterized with clinical data to mimic the inter and intra-day variability of glucose responses of 15 T1DM patients.
Results
The platform facilitates development by solving the ODE model in the cloud on large CPU-optimized machines, providing a 50% improvement in memory, speed and CPU utilization. Users can easily debug & modify code, test multiple strategies, and generate detailed clinical performance reports.
Conclusions
The platform utilizes the validated patient model to conduct virtual clinical trials for the rapid development and testing of closed-loop algorithms for T1DM.
REAL-WORLD VARIATION IN SENSOR GLUCOSE RESPONSE TO SIMILAR CARBOHYDRATE AND INSULIN
Abstract
Background and Aims
Most insulin bolus calculators model carbohydrates, insulin, and the rise in sensor glucose(SG) as a linear relationship, which does not account for meal-to-meal variations in insulin sensitivity, glycemic index, or nutrient content. The variation between carbohydrates, insulin, and SG level was evaluated in a population of real-world individuals using a sensor-augmented pump(SAP) to manage diabetes.
Methods
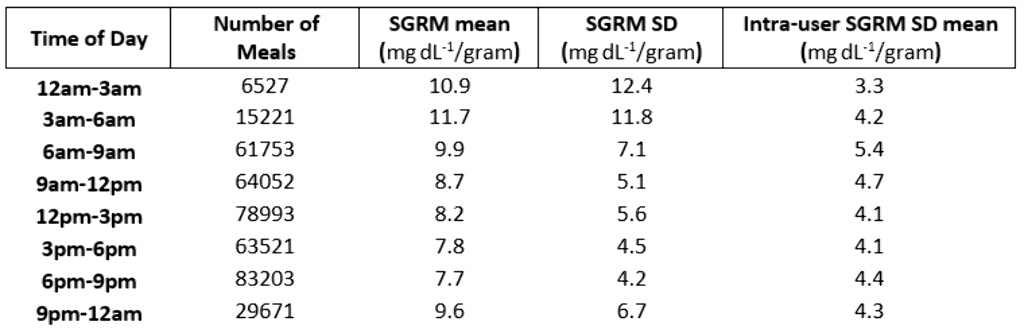
Data from 1825 SAP system users were voluntarily uploaded to CareLink™ Personal between January 2014 and October 2018 and retrospectively analyzed if ≥20 valid meals were logged. Glucose response to insulin and carbohydrates taken with a meal were measured using the Sensor Glucose Response Metric(SGRM) calculated as (SGΔ+Insulin*ISF)/Carbohydrate. SGΔ is the change in SG level from meal start to the first SG level peak. Insulin Sensitivity Factor(ISF) is the estimated glucose-to-insulin response(mg dL-1/unit). The variance in SGRM between meals users, and time of day was also determined.
Results
The mean±SD of meals for the 1825 users was 221±390. 402,941 meals were analyzed. The mean±SD of the SGRM across users was 8.4±4.3mgdL-1/gram and the average intra-user SD was 5.7mgdL-1/gram. The SGRM mean and standard deviation decreased from 3am-9pm. However, intra-user SGRM SD hit a max of 5.4mgdL-1/gram from 6am-9am.
Conclusions
The calculated SGRM of SAP therapy users revealed the potential impact of metabolic and meal-component factors(e.g., glycemic index, nutrient content, time of day, and other contextual factors) that influence glucose response in a non-linear fashion. These preliminary findings, on factors outside of carbohydrate and insulin, build toward the development of more advanced automated insulin delivery and predictive CGM systems.
DEEP REINFORCEMENT LEARNING IN THE PREDICTION OF BLOOD GLUCOSE
Abstract
Background and Aims
If individuals with diabetes want to be able to take measures in anticipation of forthcoming hypo- and hyperglycaemic events, it is essential to predict future blood glucose levels. We now report the first application of deep reinforcement learning to improve prediction of blood glucose made by a pre-trained deep neural network based on long-short term memory (LSTM).
Methods
In support of a pre-trained LSTM sequential model, an adjustment module based on Double Deep Q-Learning (DDQN) was introduced to fine tune the prediction of blood glucose. The algorithm was trained and tested on retrospectively collected data from 6 real-patient (OhioT1DM Datasets). Both LSTM and DDQN models use 4 history glucose data (look-back, sampling time 5 minutes); the former predicts a “basic” glucose value for a prediction horizon of 30 minutes, and the latter adjust, if needed, the “basic” prediction.
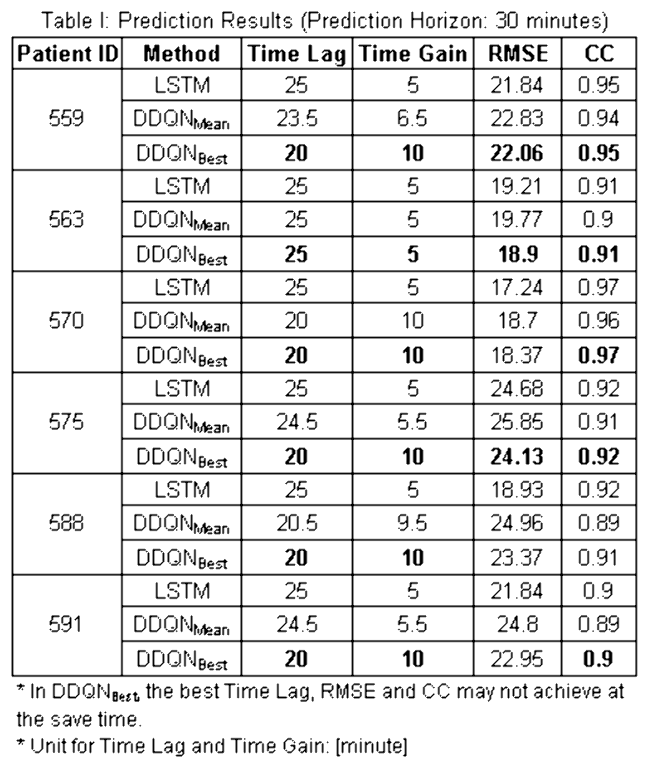
Results
As shown in Table 1, the DDQN model improved the performance of the LSTM sequential model. The Time Lags were decreased - without obvious influence on the Root Mean Square Error or the Correlation Coefficient (CC).
Conclusions
A deep reinforcement learning algorithm has been introduced to enhance the performance of a pre-trained deep LSTM network in predicting blood glucose. The preliminary results are promising, especially with respect to the delay between predicted value and real data.
EVALUATING REWARD FUNCTIONS FOR BLOOD GLUCOSE CONTROL USING REINFORCEMENT LEARNING IN THE ARTIFICIAL PANCREAS
Abstract
Background and Aims
Reinforcement learning (RL) is a promising option for adaptive and personalized algorithms for the artificial pancreas. However, when adapting reinforcement learning algorithms to new domains where a natural reward function is not given directly, such as in the T1DM case, suitable reward functions have to be crafted by hand. This design process is susceptible to errors and is in fact a general open area of research within RL.
In this work we train a single RL agent using several different reward functions in the hybrid closed loop setting. We evaluate both generic and domain-knowledge based rewards functions.
Methods
We test eight different reward functions in-silico on the Hovorka simulator. The reinforcement learning agent is trained using Trust-Region Policy Optimization (TRPO), a policy gradient algorithm that has shown previous competitive performance controlling blood glucose level in T1DM.
Performance is measured in terms of the average reward of the algorithms as well as average time in-range, -hypo and -hyper. A total of 100 days with randomized meals and fixed seed are used for generating the test averages.
Results
We test the algorithm on episodes lasting one and a half day containing four randomized meals and simulated carbohydrate counting errors. Figure 1 shows an example comparing two different reward functions - a Gaussian reward function and an asymmetric function designed to spend less time in hypoglycemia.
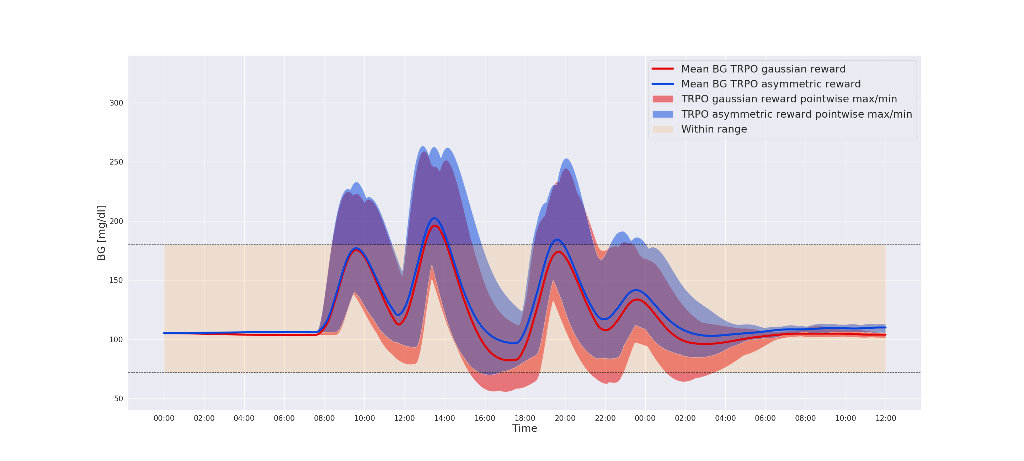
Conclusions
Our in-silico experiments shows that by tuning the reward function using domain specific knowledge of T1DM, we are able to avoid hypoglycemic events while increasing overall time-in-range.
THE USABILITY OF A HYBRID CLOSED-LOOP INSULIN DELIVERY SYSTEM: SIMULATED USE STUDIES OF THE TANDEM T:SLIM X2 INSULIN PUMP WITH CONTROL-IQ TECHNOLOGY
Abstract
Background and Aims
Advanced hybrid closed-loop (HCL) insulin delivery systems are being developed and used to minimize fluctuations in blood glucose levels associated with insulin therapy in people with diabetes. Usability of these systems is critical for their effectiveness.
The objective was to assess the usability and safety of a touchscreen, wearable insulin pump with an operating system that automatically adjusts insulin delivery based on a) predicted continuous glucose monitor (CGM) readings and b) user input that informs the pump of food intake and activity levels.
Methods
70 participants, composed of insulin pump users and MDI users, completed the usability studies. The participants were given real-life scenarios as context for simulated use tasks and knowledge tests. Tasks and tests were designed to assess the safety and ease of use of a Tandem t:slim X2 pump designed with a HCL insulin delivery system. Task success rates were collected, and a Systems Usability Scale (SUS) questionnaire was administered at the end of each session.
The formative study gave its 10 adult pump users a 10-minute orientation to the system. The summative study gave its 60 pump and MDI users real-world training, with a decay period before testing.
Results
The average SUS scores were identical at 84 indicating a high user satisfaction (typical scores 65-75). Task completion rates of 93% and 96% were observed, respectively.
Conclusions
The findings indicate that the Tandem t:slim X2 insulin pump with a hybrid closed-loop (HCL) insulin delivery system was both intuitive and safe to use.
ESTIMATION OF INSULIN ASPART AND LISPRO PEAK ACTION TIME AT POPULATION AND INDIVIDUAL LEVELS FROM INSULINEMIA MEASUREMENTS OF TYPE 1 DIABETIC PATIENTS
Abstract
Background and Aims
Insulin aspart and lispro are common analogs with a guide peak time of 1 to 3 hours. In practice, insulin action speed varies depending on formulation and patient metabolism.
In an artificial pancreas, the predicted glycaemia accuracy relies on the kinetic model of insulin subcutaneous diffusion. We aim to estimate the peak time for both analogs from insulinemia measurements Im(t).
Methods
First, the Insulin On Board (IOB) is modelled with Hovorka’s set of two sub-compartments and output plasma insulin I(t). We estimate the model’s parameters tmax, Vi and ke by minimising the quadratic error between Im(t) and I(t). The reference IOBref is equal to the sum of sub-compartments.
Then, the IOB is modelled with an exponential function IOBexp, decreasing with time and depending on τ. The optimal τ minimises the quadratic error between IOBexp(t,τ) and IOBref for all patients at once.
Results
During 24h at hospital, 41 patients with type 1 diabetes, including 20 using aspart and 21 using lispro, were monitored during clinical trials Diabeloop-SP3 and SP6.1. Measurements include CGM, basal, bolus and insulinemia Im(t).
The median individual tmax was estimated at 57 min [Q1: 49; Q3: 64] for aspart and 53 min [43; 57] for lispro. The population-optimal τ is 62 min for aspart, and 65 min for lispro.
Conclusions
In this study, insulin aspart and lispro peak times were estimated at both individual and population levels. Such values are essential to improve the glycaemia prediction accuracy by an artificial pancreas and to achieve a better diabetes management.
