John Shin, United States of America
Medtronic Clinical BiostatisticsPresenter of 2 Presentations
NIGHT TIME GLYCEMIC CONTROL BEFORE AND AFTER MINIMED™ 670G SYSTEM USE BY CHILDREN WITH T1D 2-6 YEARS OF AGE
Abstract
Background and Aims
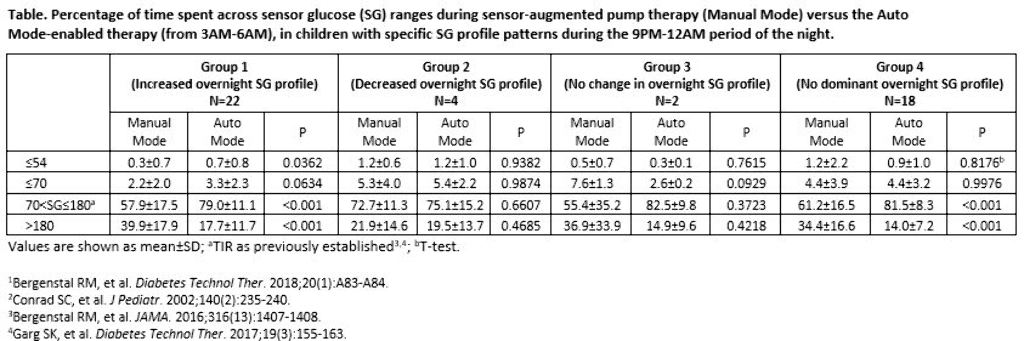
Adults who experienced increased overnight glucose variability or insulin delivery needs (akin to dawn phenomenon) during the MiniMed™ 670G system trial baseline run-in, exhibited improved overnight glycemic control after three months of Auto Mode use.1 The impact of Auto Mode use on the overnight glycemia of the youngest pediatric cohort of the MiniMed™ 670G system trials was investigated.
Methods
The 9PM-12AM sensor glucose (SG) profile of participants aged 2-6 years with T1D was analyzed, based on Conrad et al. 2002.2 Profiles were categorized as displaying a 1) >10 mg/dL increase in SG, 2) >10 mg/dL decrease in SG, or 3) No change in SG (≤10 mg/dL decrease or increase) and for >50% of the two-week baseline. If an SG profile did not present with an aforementioned pattern, it was categorized into a fourth group (No dominant SG profile). The percentage of early morning (3AM-6AM) time spent across SG levels were compared.
Results
There were 22, 4, 2, and 18 participants in Groups 1, 2, 3, and 4, respectively, during baseline. After three months of Auto Mode use, participants numbered 11, 1, 1, and 33, respectively. Significantly improved time in target glucose range (TIR, >70-180 mg/dL) was observed in Groups 1 and 4. Time spent at ≤70 mg/dL increased for Group 1, but was not significant.
Conclusions
Children with T1D displayed different profiles of overnight SG variability during the MiniMed™ 670G system trial. Regardless of the varied profile patterns observed, the MiniMed™ 670G system safely and effectively improved or did not change overnight TIR.
THE SPECTRUM OF ASSOCIATION BETWEEN HBA1C AND TIME-IN-RANGE (TIR)
Abstract
Background and Aims
There is emerging evidence of a relationship between diabetes complications and TIR which rivals that with HbA1c.1-3 Nevertheless, establishing a precise model that predicts HbA1c using TIR has been challenging and complex.4,5 The distribution of the relationship between HbA1c range and TIR was examined in participants from three MiniMed™ insulin delivery system trials.
Methods
Datasets of 211 children (7-13 years, 10.7[1.8] years) and 573 adolescents and adults (≥14 years, 41.5[17.1] years) from the three-months long MiniMed™ 670G,6,7 MiniMed™ 640G,8 and MiniMed™ 530G9 system trials were analyzed. The distributions of TIR (70-180 mg/dL) were evaluated per participant HbA1c range (<6%, 6-7%, 7.1-8%, 8.1-9%, 9.1-10%, and >10%) and R2 coefficients were calculated per TIR quintiles.
Results
A spectrum of associations between HbA1c and TIR was observed across the different trials (Figure). When data were stratified into quintiles to characterize TIR subgroups, the R2 coefficients ranged from 0.4544 (N=43) to 0.8671 (N=43) for those 7-13 years of age, and from 0.7443 (N=112) to 0.9089 (N=115) for those ≥14 years of age. Best goodness of fit was observed for participants ≥14 years.
Conclusions
The TIR quintiles from MiniMed™ insulin delivery system clinical trials demonstrated a wide variation in the degrees of glucose control within the same HbA1c range for pediatric and adult participants with type 1 diabetes. The TIR provides the quality of HbA1c that an individual patient has achieved and a basis from which to further improve glycemic control. Further studies are required to determine if TIR or HbA1c may better predict diabetes complications.

