Author Of 3 Presentations
INDIVIDUAL PULSE MONITORING AND FEEDBACK SYSTEM FOR FLASH-RT BEAM CONTROL USING FIBER-COUPLED SCINTILLATING DETECTORS
Abstract
Background and Aims
FLASH sources lack dose rate independent dosimeters and dose feedback systems. We developed an ultra-high dose rate beam monitoring system for FLASH-RT, including dose-rate independent scintillating detector and fast electronics.
Methods
A commercially available plastic scintillator and a liquid fluorescein solution were coupled to a gated integrating amplifier and a field programmable gate array (FPGA) for dose monitoring and feedback control. The FPGA was programmed to integrate dose and measure pulse width for each radiation pulse. The detectors were characterized in terms of the radiation stability, mean dose-rate (Ḋm), and dose per pulse (Dp) linearity.
Results
The Dp and the pulse width showed a consistent ramp-up period of ~4-5 pulse. The plastic scintillator was shown to be linear with Ḋm (40-380 Gy/s) and Dp (0.3-1.3 Gy/Pulse) to within ± 3%. However, the plastic scintillator was subject to significant radiation damage for the first 2 kGy (24%/kGy). The fluorescein solution was also tested to be linear with Ḋm (± 3%) and exhibited minimal radiation damage for an initial cumulative dose of 400 Gy. In-vivo dosimetry with a 1 cm circular cut-out revealed that the for the linac ramp-up period of 4 pulses, the average Dp was 0.043 ± 0.002 Gy/pulse, whereas after the ramp-up it stabilized at 0.65 ± 0.01 Gy/Pulse.


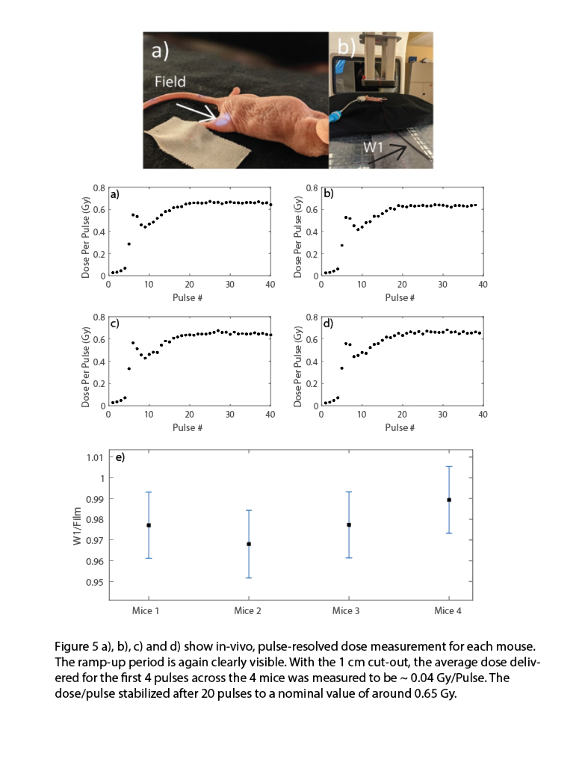
Conclusions
The tools presented in this study can be used to determine the temporal beam parameter space pertinent to the FLASH effect. Additionally, the hardware can be used to provide real-time feedback to the linac in terms of direct measurement of dose.
A RADIATION BIOLOGICAL ANALYSIS OF THE POSSIBLE MECHANISM FOR THE OXYGEN EFFECT IN FLASH
Abstract
Background and Aims
There are at least two very plausible radiobiological mechanisms for the oxygen effect in FLASH: 1) Directly, by depletion of oxygen at critical molecular sites directly changing the amount of radiation damage; 2) Indirectly by modifying physiologically mediated changes in response to radiation damage via alterations in repair and/or cell signaling. The overwhelming amount of radiation-induced damage that ultimately leads to cell death occurs in DNA. Oxygen directly radiosensitizes by reaction with transient intermediates in the DNA. Hypoxia also can modify damage from ionizing radiation inducing changes in signaling and in repair mechanisms that differ between tumors and normal tissues.
Methods
Radiobiological Principles
Results
Based on studies with cells there are lesions in DNA that have lifetimes as long as 10-5 or 10-6 seconds. The pertinent distance from which oxygen can diffuse to the sensitive site is 100-1000 nm assuming the diffusion rate of oxygen is 2.1x10-5 cm2/sec within the environment around the DNA. Therefore a technique is needed that can follow the oxygen level with spatial resolution of the nucleus and a time scale of 10-5 seconds or faster. No currently available method can do this directly. This might be done if detailed spatial distribution of oxygen inside the cell is known and the rate of oxygen depletion in a nucleus can be determined by a combination of direct measurements of oxygen, genomic alterations, and appropriate calculations.
Conclusions
Using established principles of radiation biology it should be feasible to rigorously determine if and how oxygen is involved in the mechanism of FLASH.
IN VIVO QUANTIFICATION OF OXYGEN DEPLETION BY ELECTRON FLASH IRRADIATION
Abstract
Background and Aims
The major hypothesis for the underlying mechanism of normal tissue sparing by FLASH has focused on oxygen depletion, however no experimental data have been presented to support it. The aim of this study was to assess changes in tissue oxygenation in vivo produced by FLASH irradiation.
Methods
Oxygen measurements were performed in vivo and in vitro using the phosphorescence quenching method and molecular probe Oxyphor 2P. The changes in oxygenation were quantified in response to irradiation by a 10 MeV electron beam operating at either ultra-high dose rates (UHDR) reaching 300 Gy/s or at conventional dose rates of 0.1 Gy/s.
Results
In vitro experiments with 5% BSA solutions resulted in oxygen depletion g-values of 0.19-0.21 mmHg/Gy for conventional irradiation and 0.16-0.17 mmHg/Gy for UHDR irradiation. In vivo, the total decrease in oxygen after a single fraction of 20 Gy FLASH irradiation was 2.3±0.3 mmHg in normal tissue and 1.0±0.2 mmHg in tumor tissue (p-value < 0.00001), while no changes in oxygenation were observed from a single fraction of 20 Gy applied at conventional dose rates.
Conclusions
In vitro experiments with 5% BSA solutions resulted in oxygen depletion g-values of 0.19-0.21 mmHg/Gy for conventional irradiation and 0.16-0.17 mmHg/Gy for UHDR irradiation. In vivo, the total decrease in oxygen after a single fraction of 20 Gy FLASH irradiation was 2.3±0.3 mmHg in normal tissue and 1.0±0.2 mmHg in tumor tissue (p-value < 0.00001), while no changes in oxygenation were observed from a single fraction of 20 Gy applied at conventional dose rates.

