
Presenter of 2 Presentations
CHERENKOV EXCITED LUMINESCENCE IMAGING OF DOSE AND OXYGEN IN TISSUE
Abstract
Background and Aims
During linac irradiation Cherenkov light is induced in tissue as part of the dose deposition process. This instantaneous light intensity can be used to excite molecular probes, and the signal is directly proportional to the instantaneous dose rate. Thus, during FLASH irradiation, it is possible to use this signal to sense the concentration of molecules and probes in tissue, as is investigated here.
Methods
In vivo measurement of light was done by time-gated intensified cameras that are synchronized to the linac pulses of a 10 MeV FLASH linac. Full emission spectrum light from tissue is imaged for a signal that is proportional to the Cherenkov light coming off the tissue surface. Time-delayed luminescence is imaged with different sequences of time are used to measure the emission kinetics of molecular probe Oxyphor 2P over the course of 50 microseconds after the pulse.
Results
The imaging of Cherenkov can be calibrated to dose (+/-0.5Gy accuracy) for a fixed geometry and fixed tissue optical properties. The imaging of scintillator patches is possible for quantitative dose, independent of the tissue optical properties(+/-0.1Gy accuracy). The imaging of oxygenation is possible for sensing local pO2 from up to 5 pulses of radiation to obtain a reliable lifetime (+/-1mmHg accuracy), with the camera settings used currently.
Conclusions
Pulse to pulse sensing of dose and tissue metabolism is critical to understanding the dose delivered and the tissue responses to FLASH. The tools of Cherenkov luminescence imaging can be deployed with a single time-gated camera to sense dose delivered or tissue oxygenation.
IN VIVO QUANTIFICATION OF OXYGEN DEPLETION BY ELECTRON FLASH IRRADIATION
Abstract
Background and Aims
The major hypothesis for the underlying mechanism of normal tissue sparing by FLASH has focused on oxygen depletion, however no experimental data have been presented to support it. The aim of this study was to assess changes in tissue oxygenation in vivo produced by FLASH irradiation.
Methods
Oxygen measurements were performed in vivo and in vitro using the phosphorescence quenching method and molecular probe Oxyphor 2P. The changes in oxygenation were quantified in response to irradiation by a 10 MeV electron beam operating at either ultra-high dose rates (UHDR) reaching 300 Gy/s or at conventional dose rates of 0.1 Gy/s.
Results
In vitro experiments with 5% BSA solutions resulted in oxygen depletion g-values of 0.19-0.21 mmHg/Gy for conventional irradiation and 0.16-0.17 mmHg/Gy for UHDR irradiation. In vivo, the total decrease in oxygen after a single fraction of 20 Gy FLASH irradiation was 2.3±0.3 mmHg in normal tissue and 1.0±0.2 mmHg in tumor tissue (p-value < 0.00001), while no changes in oxygenation were observed from a single fraction of 20 Gy applied at conventional dose rates.
Conclusions
In vitro experiments with 5% BSA solutions resulted in oxygen depletion g-values of 0.19-0.21 mmHg/Gy for conventional irradiation and 0.16-0.17 mmHg/Gy for UHDR irradiation. In vivo, the total decrease in oxygen after a single fraction of 20 Gy FLASH irradiation was 2.3±0.3 mmHg in normal tissue and 1.0±0.2 mmHg in tumor tissue (p-value < 0.00001), while no changes in oxygenation were observed from a single fraction of 20 Gy applied at conventional dose rates.
Author Of 6 Presentations
INDIVIDUAL PULSE MONITORING AND FEEDBACK SYSTEM FOR FLASH-RT BEAM CONTROL USING FIBER-COUPLED SCINTILLATING DETECTORS
Abstract
Background and Aims
FLASH sources lack dose rate independent dosimeters and dose feedback systems. We developed an ultra-high dose rate beam monitoring system for FLASH-RT, including dose-rate independent scintillating detector and fast electronics.
Methods
A commercially available plastic scintillator and a liquid fluorescein solution were coupled to a gated integrating amplifier and a field programmable gate array (FPGA) for dose monitoring and feedback control. The FPGA was programmed to integrate dose and measure pulse width for each radiation pulse. The detectors were characterized in terms of the radiation stability, mean dose-rate (Ḋm), and dose per pulse (Dp) linearity.
Results
The Dp and the pulse width showed a consistent ramp-up period of ~4-5 pulse. The plastic scintillator was shown to be linear with Ḋm (40-380 Gy/s) and Dp (0.3-1.3 Gy/Pulse) to within ± 3%. However, the plastic scintillator was subject to significant radiation damage for the first 2 kGy (24%/kGy). The fluorescein solution was also tested to be linear with Ḋm (± 3%) and exhibited minimal radiation damage for an initial cumulative dose of 400 Gy. In-vivo dosimetry with a 1 cm circular cut-out revealed that the for the linac ramp-up period of 4 pulses, the average Dp was 0.043 ± 0.002 Gy/pulse, whereas after the ramp-up it stabilized at 0.65 ± 0.01 Gy/Pulse.


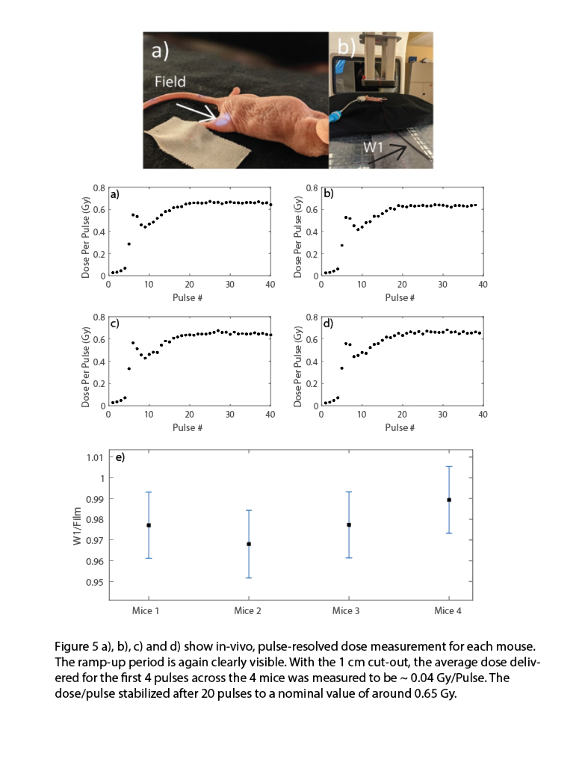
Conclusions
The tools presented in this study can be used to determine the temporal beam parameter space pertinent to the FLASH effect. Additionally, the hardware can be used to provide real-time feedback to the linac in terms of direct measurement of dose.
LONGITUDINAL IN-VIVO ASSESSMENT OF MOUSE SKIN DAMAGE WITH FUNCTIONAL OPTICAL COHERENCE TOMOGRAPHY IN FLASH VERSUS CONVENTIONAL RADIOTHERAPY
Abstract
Background and Aims
Reduced skin toxicity is now shown pre-clinically after >40Gy/s FLASH radiotherapy (RT) as compared to ~0.03Gy/s conventional RT. While this area of study has been advanced significantly, the biological basis of the FLASH sparing effect on skin is yet to be discovered, and diagnostic tools to assess the response biological mechanisms and tools that can be used in the translation of FLASH study in humans are needed. Here we report on direct mechanistic in situ measurements of skin damage, with the aim to quantify and compare microstructural and microvascular changes in skin after RT, while varying dose and supporting the findings with ex vivo histological observations.
Methods
In IACUC approved animal study, right thighs of 54 nude mice (n=6 per dose group) were treated with 0-40Gy single doses of 300Gy/s FLASH and conventional RT. Skin was imaged with functional optical coherence tomography - a non-invasive microscopic 3D imaging technique with light penetration at 1-3 millimeter depths in biological tissues.
Results
Quantification was completed for skin pigmentation, epidermal thickness, remodeling of collagen fibers, alteration of blood and lymphatic networks. These demonstrated inter-connected “passenger versus driver” temporal behaviors of skin tissue components in both RT-treatment types. Response to FLASH RT was characterized by reduced damage to collagen bundles and blood/lymphatic networks together with less desquamation of skin surface (less damage to epidermis) and higher pigmentation.
Conclusions
This study presents first of its kind in-vivo functional longitudinal observations and quantification of the comparative FLASH effects in a mouse model of skin early damage, healing and recovery.
ELECTRON FLASH FOR THE CLINIC: LINAC CONVERSION, COMMISSIONING AND TREATMENT PLANNING
Abstract
Background and Aims
We present the rigorous commissioning of a modified LINAC to deliver ultrahigh dose-rate (UHDR) electron beam and implementation of its model in a widely adopted treatment planning system (TPS) with minimal changes to the clinical setting.
Methods
A Varian Clinac 2100C/D was converted to deliver UHDR beams by withdrawing the target and scattering foil in 10MV x-ray mode. Beam characteristics and stability were quantified by film, Cherenkov, and scintillation imaging. The Geant4 generated beam model was validated with film and implemented in Varian Eclipse TPS. Electron FLASH radiotherapy (eFLASH-RT) plans were generated for representative mammal and human patient cases accounting for complex geometries and anatomical inhomogeneities.
Results
The surface mean-dose-rate at the isocenter was >230Gy/s for all measured fields with adequate long-term stability (deviations of output <7%, symmetry/flatness <2%, spatial shift and FWHM <2mm). The TPS model was validated to clinical accuracy (average error <1.5% for lateral profiles and <2% for percent-depth-dose profiles). Treatments plans were generated and accurately delivered to normal porcine skin surface tissue and a melanoma tumor in a canine’s posterior oral cavity. A human eFLASH-RT plan comparable to a conventional electron plan was achieved by utilizing routine accessories, oblique gantry angle and couch kick.


Conclusions
Treatment planning and accurate delivery of eFLASH-RT were feasible in minimally modified radiation oncology clinical settings. The modifications and open-source TPS model are readily transferable to facilitate clinical translation of eFLASH-RT.
Acknowledgment: This work was supported by the Norris Cotton Cancer Center (grant P30CA023108) and Thayer School of Engineering (seed funding and grant R01EB024498).
CHERENKOV EXCITED LUMINESCENCE IMAGING OF DOSE AND OXYGEN IN TISSUE
Abstract
Background and Aims
During linac irradiation Cherenkov light is induced in tissue as part of the dose deposition process. This instantaneous light intensity can be used to excite molecular probes, and the signal is directly proportional to the instantaneous dose rate. Thus, during FLASH irradiation, it is possible to use this signal to sense the concentration of molecules and probes in tissue, as is investigated here.
Methods
In vivo measurement of light was done by time-gated intensified cameras that are synchronized to the linac pulses of a 10 MeV FLASH linac. Full emission spectrum light from tissue is imaged for a signal that is proportional to the Cherenkov light coming off the tissue surface. Time-delayed luminescence is imaged with different sequences of time are used to measure the emission kinetics of molecular probe Oxyphor 2P over the course of 50 microseconds after the pulse.
Results
The imaging of Cherenkov can be calibrated to dose (+/-0.5Gy accuracy) for a fixed geometry and fixed tissue optical properties. The imaging of scintillator patches is possible for quantitative dose, independent of the tissue optical properties(+/-0.1Gy accuracy). The imaging of oxygenation is possible for sensing local pO2 from up to 5 pulses of radiation to obtain a reliable lifetime (+/-1mmHg accuracy), with the camera settings used currently.
Conclusions
Pulse to pulse sensing of dose and tissue metabolism is critical to understanding the dose delivered and the tissue responses to FLASH. The tools of Cherenkov luminescence imaging can be deployed with a single time-gated camera to sense dose delivered or tissue oxygenation.
A RADIATION BIOLOGICAL ANALYSIS OF THE POSSIBLE MECHANISM FOR THE OXYGEN EFFECT IN FLASH
Abstract
Background and Aims
There are at least two very plausible radiobiological mechanisms for the oxygen effect in FLASH: 1) Directly, by depletion of oxygen at critical molecular sites directly changing the amount of radiation damage; 2) Indirectly by modifying physiologically mediated changes in response to radiation damage via alterations in repair and/or cell signaling. The overwhelming amount of radiation-induced damage that ultimately leads to cell death occurs in DNA. Oxygen directly radiosensitizes by reaction with transient intermediates in the DNA. Hypoxia also can modify damage from ionizing radiation inducing changes in signaling and in repair mechanisms that differ between tumors and normal tissues.
Methods
Radiobiological Principles
Results
Based on studies with cells there are lesions in DNA that have lifetimes as long as 10-5 or 10-6 seconds. The pertinent distance from which oxygen can diffuse to the sensitive site is 100-1000 nm assuming the diffusion rate of oxygen is 2.1x10-5 cm2/sec within the environment around the DNA. Therefore a technique is needed that can follow the oxygen level with spatial resolution of the nucleus and a time scale of 10-5 seconds or faster. No currently available method can do this directly. This might be done if detailed spatial distribution of oxygen inside the cell is known and the rate of oxygen depletion in a nucleus can be determined by a combination of direct measurements of oxygen, genomic alterations, and appropriate calculations.
Conclusions
Using established principles of radiation biology it should be feasible to rigorously determine if and how oxygen is involved in the mechanism of FLASH.
IN VIVO QUANTIFICATION OF OXYGEN DEPLETION BY ELECTRON FLASH IRRADIATION
Abstract
Background and Aims
The major hypothesis for the underlying mechanism of normal tissue sparing by FLASH has focused on oxygen depletion, however no experimental data have been presented to support it. The aim of this study was to assess changes in tissue oxygenation in vivo produced by FLASH irradiation.
Methods
Oxygen measurements were performed in vivo and in vitro using the phosphorescence quenching method and molecular probe Oxyphor 2P. The changes in oxygenation were quantified in response to irradiation by a 10 MeV electron beam operating at either ultra-high dose rates (UHDR) reaching 300 Gy/s or at conventional dose rates of 0.1 Gy/s.
Results
In vitro experiments with 5% BSA solutions resulted in oxygen depletion g-values of 0.19-0.21 mmHg/Gy for conventional irradiation and 0.16-0.17 mmHg/Gy for UHDR irradiation. In vivo, the total decrease in oxygen after a single fraction of 20 Gy FLASH irradiation was 2.3±0.3 mmHg in normal tissue and 1.0±0.2 mmHg in tumor tissue (p-value < 0.00001), while no changes in oxygenation were observed from a single fraction of 20 Gy applied at conventional dose rates.
Conclusions
In vitro experiments with 5% BSA solutions resulted in oxygen depletion g-values of 0.19-0.21 mmHg/Gy for conventional irradiation and 0.16-0.17 mmHg/Gy for UHDR irradiation. In vivo, the total decrease in oxygen after a single fraction of 20 Gy FLASH irradiation was 2.3±0.3 mmHg in normal tissue and 1.0±0.2 mmHg in tumor tissue (p-value < 0.00001), while no changes in oxygenation were observed from a single fraction of 20 Gy applied at conventional dose rates.

