Browsing Over 510 Presentations
2.0.4 - Redefine the Goals of Cartilage Repair – What is Next?
3.1.1 - Arthroscopic Approach to Cartilage Defects in The Knee (Pre-Recorded)
3.1.2 - Arthroscopic Approach to Cartilage Defects – Ankle (Pre-Recorded)
Abstract
Introduction
A number of operative treatment options have been reported to treat osteochondral lesions of the talus (OLT). The choice of operative treatment methods are dependent on the grade, size and site of the lesion. Recently, several new techniques have been introduced for the management of such lesions. However, there is a lack randomized or comparative studies to determine whether one technique is superior to the others. Bone marrow stimulation with microfracture arthroplasty is the most common procedure for the management of OLT. It induces repair of localized articular cartilage defects of the talus. The use of additional scaffolds has become increasingly popular with several case series reporting promising results in the treatment of OLT´s in athletes and with the combination of an autologous bone graft in the mid-term.
Content
The treatment with a scaffold has been designed to be performed by an open technique that often involves a medial or lateral malleolar osteotomy. Arthroscopic treatment is believed being advantageous because it is associated with significantly less surgical trauma than the open technique, and it avoids the need for a malleolar osteotomy. Furthermore, arthroscopic evaluation allows the status of the cartilage to be accurately assessed in all parts of the joint. Possible complications of malleolus osteotomy that include direct morbidity by injury to adjacent structures, mid-term morbidity by malunion or non-union of the osteotomy, and long-term morbidity by the development of local cartilage degeneration and the need for hardware removal, which may become symptomatic in an area with a limited soft tissue envelope, can be avoided.
The procedure is performed with the patient under either spinal or general anesthesia in the supine position using standard anteromedial and anterolateral portal approaches for the ankle. The application of a thigh tourniquet at a pressure of 250-300 mmHg is recommended. For distraction, a Hintermann-Spreader or an ankle arthroscopy distraction strap or bandage can be used. Debridement of the lesion is performed in common fashion by creating healthy and stable cartilage borders and vital bleeding subchondral bone by bone marrow stimulation. If a bone graft is needed (recommended in lesions with a depth of >3mm bone defect), enlargement of the portal, by which the lesion is treated, is recommended to a length of about 2cm. The intraarticular fluid needs to be removed after debridement and the OLT dried using cotton sponges. The application of a cannula might facilitate the application of the bone graft and an acellular matrix. Fixation of the matrix might be necessary using a commercially available fibrin glue. The stability of the matrix should be checked within a normal ankle range of motion under direct arthroscopic vision.
Reported complication rates for the arthroscopic approach in the treatment of OLT of the talus are low. Arthroscopic bone marrow stimulation with microfracture alone and with the addition of an acellular matrix with or without a bone graft are effective for the treatment of patients suffering from osteochondral talar lesions. Further research is requested to determine possible differences with respect to possible influencing parameters such as lesion size and its depth.
3.1.3 - The Hip
3.1.4 - The Shoulder & Elbow
Abstract
Introduction
The forces applied to the cartilage surfaces of the shoulder and elbow are generally lower than at the weightbearing joints of the lower extremities. Thus, focal chondral or osteochondral defects and early osteoarthritis are considerably less common than in the knee, hip or ankle.
Nonetheless, potentially clinically relevant focal defects can be found in over one fifth of patients with rotator cuff tears, impingement or general shoulder pain. The reasons for focal glenohumeral cartilage defects are manifold and include previous surgeries, trauma, osteochodritis dissecans, infections, avascular necrosis, rheumatoid arthritis, instabilities, or rotator cuff insufficiencies.
Content
The symptoms of glenohumeral cartilage defects include stress-induced shoulder pain or pain at rest, limited sports performance, sharp pain or mechanical obstructions, or progressively increasing pain. However, symptoms are often unspecific and frequently do not allow to diagnose cartilage defects or gauge their significance in shoulder pain. Thus, the treatment of concomitant pathologies is a primary goal when glenohumeral cartilage defects are diagnosed and treated. As cartilage lesions around the shoulder are often an incidental finding and rarely occur in isolation, the influence on outcomes of treating concomitant pathhologies versus addressing cartilage defects is still controversial.
Various treatment options are available today, whereas overall results are very promising independent of chosen treatment modality. According to personal experience and scientific evidence, microfracturing is a simple but reliable treatment option, which leads to improvements of shoulder function and pain reduction. In contrast to microfracturing in the knee, results tend to remain stable even in the longterm. Similar to the knee, microfracturing is the first line treatment for focal, chondral defects of the glenohumeral joint under 2cm2. Larger chondral defects (2-6cm2) can be addressed with various forms of autologous chondrocyte transplantation. If there is bone involvement present (osteochondral lesions), osteochondral transfers from the humerus or knee provide a treatment option for defects <2.5cm2. Larger osteochondral lesions can be treated with partial humeral head resurfacing.
Around the elbow, focal chondral and osteochondral lesions are particularly common at the humeral capitulum in the context of an osteochondrosis dissecans in gymnasts and repetitive overhead or throwing athletes. Osteochondral lesions of the elbow also occur as the result of acute trauma or reduced joint congruency due to instability.
Contrary to the shoulder, microfracturing has not been as reliable around the elbow and has been associated with progressive degeneration and low return to sports rates. Thus, it should be reserved for small defects with limited symptoms when encountered as an incidental finding during arthroscopy for concomitant pathologies. For larger chondral lesions or osteochondral defects, autologous osteochondral transplantation - primarily using osteochondral plugs from the ipsilateral proximal femoral condyle - is the treatment option of choice. This has shown relatively good clinical and radiological results even in the longterm, while progression of degeneration seems to be slower compared to microfracturing. Furthermore, due to its anatomic characteristics and congruent nature of the elbow joint, treatment options are significantly influenced by the exact location of the defect. Treatment of concomitant pathologies (e.g. instabilities, plicae, loose bodies, etc.) and restoration of native joint mechanics are especially important at the elbow.
3.2.1 - Long-Term Results
Abstract
Introduction
Several surgical strategies have been investigated to address cartilage lesions, ranging from classic microfracturing to the most recent cell-based therapies or cell-free approaches based on “smart” biomimetic scaffolds. Treatments such as mosaicplasty, autologous or allogeneic osteochondral transplantation, autologous chondrocyte implantation, matrix-assisted autologous chondrocyte transplantation (MACT), and osteochondral scaffolds can all be considered as possible treatments for cartilage pathology. However, each technique has advantages and limits. Therefore, surgeons should evaluate every option before deciding how to proceed. In particular, while most new approaches seem to hold promise, it is important to understand their real potential by focusing on the long-term results.
Content
Expensive high-tech procedures are often available only in highly specialized centers and are not an option for many orthopedic surgeons. Therefore, besides the fashionable and ambitious regenerative treatments, it is important to start by evaluating also the less expensive and easily available treatments to address the damaged osteochondral unit. Among these, mosaicplasty and single-plug autologous osteochondral transplantation are viable solutions. These approaches present some analogies in terms of treatment rationale and technical aspects. Both procedures consist of harvesting autologous osteochondral grafts from a healthy region of the knee to be placed in the defect site. Looking at the long-term results of single-plug autologous osteochondral transplantation, we documented in a series of 15 patients studied at minimum 16 years’ follow-up a significant improvement and only 3 failures, although the absolute scores reached were only moderately satisfactory [1].
Mosaicplasty is a less invasive approach, which can be performed also arthroscopically, with the possibility of harvesting multiple small cylinder-shaped autografts from different articular sites. This technique is less invasive and contribute to less donor site morbidity, which in theory should allow for the treatment of larger lesions. Still, while showing overall good clinical results, our analysis of 26 patients followed up to 12 years of follow-up, the radiographic evaluation showed significantly poorer Kellgren-Lawrence scores and a reduction of the joint line in the treated compartments, and knees with 3-4 plugs presented a significantly higher joint degeneration level with respect to those implanted with 1-2 plugs [2]. The comparison of mosaicplasty and MACT showed in both cases a clinical improvement, however, for larger lesions MACT presented better subjective and objective outcomes, as well as less failures, which should be considered when choosing the most suitable treatment for patients affected by knee cartilage lesions [3].
MACT showed actually really good results over time. A long-term evaluation of 113 patients documented a long-lasting improvement that was stable over time and resulted in a limited number of failures and reinterventions for up to 15 years of follow-up. However, not all patients benefited in the same way, and several factors were identified as having a prognostic value [4]. Arthroscopic bone grafting followed by MACT for unfixable knee osteochondritis dissecans (OCD) can offer a promising and stable clinical outcome over time especially in lesions smaller than 4 cm2, with a low failure rate, but persistent subchondral alterations were documented at long-term MRI evaluation, suggesting the limits of this approach to regenerate the osteochondral unit in patients affected by knee OCD [5,6]. More challenging are the patello-femoral lesions, which provide overall lower results than condyle lesions, even though the improvement achieved was relatively stable at long-term. The clinical results of hyaluronan-based MACT treatment of chondral lesions of the patellofemoral joint did not worsen over time in our series, with an overall low rate of failure at long-term follow-up [7]. Still, these overall findings may be misleading. In fact, while commonly studied together in the group of patello-femoral lesions, patient characteristics differ between patellar and trochlear cartilage defects. Moreover, the results obtained are significantly different, with a markedly good outcome in cases with trochlear lesions and less satisfactory results for patients affected by cartilage lesions of the patella. Thus, patellar and trochlear defects should be considered separately when evaluating the outcome of cartilage treatments in this anatomic region [8].
MACT was also used as salvage procedures for young, active patients affected by chondral and osteochondral lesions in osteoarthritic knees. This led to a limited improvement, with the majority of patients experiencing failure at long-term follow-up, for a total surgical and clinical failure rate of 59% at 15 years. Although a minor subpopulation experienced favorable and stable improvement, the use of MACT for such a challenging indication remains questionable until responding patients can be profiled [9].
For degenerative lesions, as well as for large OCDs, treatments should also aim at addressing the damaged subchondral bone. A cell-free biomimetic osteochondral scaffold has been developed to this purpose. The 10-year follow-up evaluation showed that the clinical improvement was significant and stable over time both in terms of subjective and objective outcomes, including activity level, with overall good results. On the other hand, the regenerative potential of this scaffold is limited, as demonstrated by the signal alterations persisting over time on MRI scans [10]. Thus, while the clinical improvement is still relevant and stable over time, efforts are being placed to further improve the biological potential of this osteochondral scaffold. An ongoing Eu project is investigating both chemical and biological strategies to enhance the subchondral bone regenerative potential, with promising results in the animal model [11].
The partial results obtained by these new treatments aiming at addressing challenging lesions of the articular surface are also explainable by an unfavorable joint environment, which should be addressed as well. This is a rapidly growing field, where increasing efforts have been invested to develop orthobiologics to positively modulate the articular homeostasis. Among these, platelet-rich plasma (PRP) is the most used and documented. A meta-analysis of 34 RCTs showed that the effect of platelet concentrates goes beyond its mere placebo effect, and PRP injections provide better results than other injectable options. This benefit increases over time, being not significant at earlier follow-ups but becoming clinically significant after 6 to 12 months [12]. However, although substantial, the improvement remains partial and supported by low level of evidence. Among the controversial aspects, the long-term results of such injections remain poorly documented. Few reports beyond 2 years follow-up report an overall declining outcome [13,14]. Moreover, results are even less successful when addressing the more demanding middle-aged sport-active patients with knee osteoarthritis, which showed only a limited return to sport [15]. Thus, while a recent systematic review [16] documented that platelet-rich plasma injections induce disease-modifying effects in the treatment of osteoarthritis in animal models, results in human are still modest, and further research efforts are needed to optimize the potential of platelet concentrates, as well as to identify the ideal candidates that could benefit more by these emerging orthobiologic approaches.
References
1) Filardo G, Kon E, Di Matteo B, Di Martino A, Marcacci M. Single-plug autologous osteochondral transplantation: results at minimum 16 years' follow-up. Orthopedics. 2014 Sep;37(9):e761-7. doi: 10.3928/01477447-20140825-51.
2) Filardo G, Kon E, Perdisa F, Tetta C, Di Martino A, Marcacci M. Arthroscopic mosaicplasty: long-term outcome and joint degeneration progression. Knee. 2015 Jan;22(1):36-40. doi: 10.1016/j.knee.2014.10.001.
3) Zaffagnini S, Boffa A, Andriolo L, Reale D, Busacca M, Di Martino A, Filardo G. Mosaicplasty versus Matrix-Assisted Autologous Chondrocyte Transplantation for Knee Cartilage Defects: A Long-Term Clinical and Imaging Evaluation. Appl Sci. 2020 Jul 3; 10(13), 4615; doi.org/10.3390/app10134615.
4) Andriolo L, Reale D, Di Martino A, De Filippis R, Sessa A, Zaffagnini S, Filardo G. Long-term Results of Arthroscopic Matrix-Assisted Autologous Chondrocyte Transplantation: A Prospective Follow-up at 15 Years. Am J Sports Med. 2020 Oct;48(12):2994-3001. doi: 10.1177/0363546520949849.
5) Andriolo L, Di Martino A, Altamura SA, Boffa A, Poggi A, Busacca M, Zaffagnini S, Filardo G. Matrix-assisted chondrocyte transplantation with bone grafting for knee osteochondritis dissecans: stable results at 12 years. Knee Surg Sports Traumatol Arthrosc. 2021 Jun;29(6):1830-1840. doi: 10.1007/s00167-020-06230-y.
6) Roffi A, Andriolo L, Di Martino A, Balboni F, Papio T, Zaffagnini S, Filardo G. Long-term Results of Matrix-assisted Autologous Chondrocyte Transplantation Combined With Autologous Bone Grafting for the Treatment of Juvenile Osteochondritis Dissecans. J Pediatr Orthop. 2020 Feb;40(2):e115-e121.
7) Kon E, Filardo G, Gobbi A, Berruto M, Andriolo L, Ferrua P, Crespiatico I, Marcacci M. Long-term Results After Hyaluronan-based MACT for the Treatment of Cartilage Lesions of the Patellofemoral Joint. Am J Sports Med. 2016 Mar;44(3):602-8. doi: 10.1177/0363546515620194.
8) Filardo G, Kon E, Andriolo L, Di Martino A, Zaffagnini S, Marcacci M. Treatment of "patellofemoral" cartilage lesions with matrix-assisted autologous chondrocyte transplantation: a comparison of patellar and trochlear lesions. Am J Sports Med. 2014 Mar;42(3):626-34. doi: 10.1177/0363546513510884.
9) Andriolo L, Reale D, Di Martino A, Zaffagnini S, Vannini F, Ferruzzi A, Filardo G. High Rate of Failure After Matrix-Assisted Autologous Chondrocyte Transplantation in Osteoarthritic Knees at 15 Years of Follow-up. Am J Sports Med. 2019 Jul;47(9):2116-2122. doi: 10.1177/0363546519855029.
10) Di Martino A, Perdisa F, Filardo G, Busacca M, Kon E, Marcacci M, Zaffagnini S. Cell-Free Biomimetic Osteochondral Scaffold for the Treatment of Knee Lesions: Clinical and Imaging Results at 10-Year Follow-up. Am J Sports Med. 2021 Aug;49(10):2645-2650. doi: 10.1177/03635465211029292.
11) EuroNanoMed III Project: this project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no 723770. Funding was also provided by the following funding organisations (Science Foundation of Ireland, Ireland, Grant Number SFI/16/ENM-ERA/3458, Ministero della Salute (IMH), Italy, Stated Education Development Agency SEDA/VIAA, Latvia, Technology Foundation (STW), The Netherlands).
12) Filardo G, Previtali D, Napoli F, Candrian C, Zaffagnini S, Grassi A. PRP Injections for the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. Cartilage. 2020 Jun 19:1947603520931170
13) Kon E, Engebretsen L, Verdonk P, Nehrer S, Filardo G. Autologous Protein Solution Injections for the Treatment of Knee Osteoarthritis: 3-Year Results. Am J Sports Med. 2020 Sep;48(11):2703-2710. doi: 10.1177/0363546520944891.
14) Di Martino A, Di Matteo B, Papio T, Tentoni F, Selleri F, Cenacchi A, Kon E, Filardo G. Platelet-Rich Plasma Versus Hyaluronic Acid Injections for the Treatment of Knee Osteoarthritis: Results at 5 Years of a Double-Blind, Randomized Controlled Trial. Am J Sports Med. 2019 Feb;47(2):347-354. doi: 10.1177/0363546518814532.
15) Altamura SA, Di Martino A, Andriolo L, Boffa A, Zaffagnini S, Cenacchi A, Zagarella MS, Filardo G. Platelet-Rich Plasma for Sport-Active Patients with Knee Osteoarthritis: Limited Return to Sport. Biomed Res Int. 2020 Jan 31;2020:8243865. doi: 10.1155/2020/8243865.
16) Boffa A, Salerno M, Merli G, De Girolamo L, Laver L, Magalon J, Sánchez M, Tischer T, Filardo G. Platelet-rich plasma injections induce disease-modifying effects in the treatment of osteoarthritis in animal models. Knee Surg Sports Traumatol Arthrosc. 2021 Aug 2. doi: 10.1007/s00167-021-06659-9.
3.2.2 - Registry Data
3.2.3 - MSCs: Where is the Evidence?
Abstract
Introduction
Injury of the knee articular cartilage and osteochondral unit is a significant cause of functional limitation in which the goal of treatment is preservation of the native knee joint. There are a variety of cell-based cartilage and osteochondral unit repair methods that may be used to treat different injuries, given the limited potential for cartilage injury to heal without intervention. Restoration of hyaline-like cartilage is the ultimate goal in treatment of osteochondral unit as it offers an improved durability of repaired tissue and preferable wear characteristics. Over the years many cell-based therapies have been developed to address the need for the long-term viability of repaired tissue. Some of the techniques use mesenchymal stem cells (MSCs) as a core ingredient for tissue repair.
Content
Microfracture, a bone marrow stimulation technique using MSCs, when used wisely and with caution in selected patients has shown good clinical results at short-term follow up. Nonetheless, deterioration of the clinical outcome may be expected after 2 -3 years post-treatment, and degenerative changes are present at long-term follow-up, with a higher rate in older patients with extensive and multiple lesions [1, 2]. Autologous Matrix-Induced Chondrogenesis (AMIC) has emerged as a modification of the microfracture technique by addition of a collagen scaffold. However both of these techniques raise a concern of the damage of the subchondral bone and the formation of microcysts, that may quicken the deterioration of the cartilage and compromise the articular surface for future procedures [3, 4]. Autologous chondrocyte implantation (ACI) consists of a two-step procedure; first, a sample of healthy cartilage is harvested from a non-weight bearing site, followed by an in vitro cell expansion. The second step is the implantation of the chondrocyte suspension into the cartilage defect. Compared to bone marrow stimulating techniques such as microfracture, ACI technique has appeared to be superior over time due to longer-lasting effects, without the concerns of destruction of the subchondral bone [5,6]. However, while techniques using autologous chondrocytes have demonstrated durable cartilage repair, these methods require the patient to undergo two surgical procedures due to the need for chondrocyte culture.
Hyaluronic acid-based scaffold with bone marrow aspirate concentrate (HA-BMAC) was developed 30 years ago, it allowed the treatment of larger cartilage defects in a one-step surgery with biologic tissue such as mesenchymal stem cells, chondrocytes, or platelet-rich plasma. This technique has provided long-term results and has proven its superiority to microfracture due to lasting effect over ten years compared to the 2-3 years with microfracture technique [2]. Moreover, it can be used even in cases of multiple compartment injury, extensive lesions, or in older patients [7-10]. This procedure provides a good source of chondrocytes, whether directly or through differentiation of multipotent precursor cells, capable of producing hyaline-like cartilage, with minimal formation of fibrocartilage tissue [10].
Lately more attention was brought to the osteochondral unit and especially the role of subchondral bone in maintaining homeostasis of the joint [4]. Bone marrow lesions (BMLs) are the focal changes in the subchondral bone and can be identified by magnetic resonance imaging (MRI). A technique using MSCs to treat BMLs, the Osteo-Core-Plasty (Marrow Cellution™) is a minimally invasive subchondral bone augmentation that offers both biologic and structural components to optimize the osteochondral environment for regeneration. This technique may also by applied in treatment of insufficiency fractures, subchondral cysts, and avascular necrosis [11, 12].
MSCs are also found in other sources than bone marrow, one of which is fat tissue. Adipose derived Mesenchymal Stem Cells (ADMSCs) are quickly becoming a viable source of MSC, not only because they are easy to harvest but also have a high concentration of progenitor cells. Primary outcomes of the use of microfragmented adipose tissue (MFAT) injection in elderly patients with knee osteoarthritis (OA) have shown good clinical results compared with the pre-treatment state and could be an alternative treatment for elderly patients 60 years or older [13, 14].
Summarizing, over 30 years of using MSCs have shown good and very good clinical outcomes in treatment of osteochondral unit lesions and OA.
References
References:
1. Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014 Sep;22(9):1986–96.
2. Gobbi A, Whyte GP. One-Stage Cartilage Repair Using a Hyaluronic Acid-Based Scaffold With Activated Bone Marrow-Derived Mesenchymal Stem Cells Compared With Microfracture: Five-Year Follow-up. Am J Sports Med. 2016 Nov;44(11):2846–54.
3. Frank RM, Cotter EJ, Nassar I, Cole B. Failure of Bone Marrow Stimulation Techniques. Sports Med Arthrosc Rev. 2017 Mar;25(1):2–9.
4. Gobbi A, Alvarez R, Irlandini E, Dallo I. Current Concepts in Subchondral Bone Pathology. In: Gobbi A, Lane JG, Longo UG, Dallo I, editors. Joint Function Preservation: A Focus on the Osteochondral Unit [Internet]. Cham: Springer International Publishing; 2022
5. Gobbi A, Lane JG, Dallo I. Editorial Commentary: Cartilage Restoration-What Is Currently Available? Arthroscopy. 2020 Jun;36(6):1625–8.
6. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994 Oct 6;331(14):889–95.
7. Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014 Mar;42(3):648–57.
8. Gobbi A, Karnatzikos G, Scotti C, Mahajan V, Mazzucco L, Grigolo B. One-Step Cartilage Repair with Bone Marrow Aspirate Concentrated Cells and Collagen Matrix in Full-Thickness Knee Cartilage Lesions: Results at 2-Year Follow-up. Cartilage. 2011 Jul;2(3):286–99.
9. Gobbi A, Scotti C, Karnatzikos G, Mudhigere A, Castro M, Peretti GM. One-step surgery with multipotent stem cells and Hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years. Knee Surg Sports Traumatol Arthrosc. 2017 Aug;25(8):2494–501.
10. Gobbi A, Whyte GP. Long-term Clinical Outcomes of One-Stage Cartilage Repair in the Knee With Hyaluronic Acid-Based Scaffold Embedded With Mesenchymal Stem Cells Sourced From Bone Marrow Aspirate Concentrate. Am J Sports Med. 2019 Jun;47(7):1621–8.
11. Szwedowski D, Dallo I, Irlandini E, Gobbi A. Osteo-core Plasty: A Minimally Invasive Approach for Subchondral Bone Marrow Lesions of the Knee. Arthrosc Tech. 2020 Nov;9(11):e1773–7
12. Gobbi A, Dallo I. Osteo-Core-Plasty technique for the treatment of a proximal tibial subchondral cystic lesion. 2021;
13. Dallo I, Morales M, Gobbi A. Platelets and Adipose Stroma Combined for the Treatment of the Arthritic Knee. Arthroscopy Techniques. 2021 Oct 6;10.
14. Gobbi A, Dallo I, Rogers C, Striano RD, Mautner K, Bowers R, et al. Two-year clinical outcomes of autologous microfragmented adipose tissue in elderly patients with knee osteoarthritis: a multi-centric, international study. Int Orthop. 2021 May;45(5):1179–88.
3.2.4 - Guidelines for Cartilage Repair (Recorded Presentation)
Abstract
Introduction
There is a noteworthy difference between the breadth and depth of data on cartilage repair techniques and their clinical use. Despite more than three decades of accumulated supporting information, most cartilage repair options are still rarely encountered outside the specialized community.
Content
One reason for this are the problems associated with navigating the data to chose an appropriate treatment for the patient at hand. In order to facilitate this process, individual data points are being assembled to evidence, as seen in the prior talks in the session. This evidence can be thought of as the “map to cartilage repair”
However, true to this analogy, a map alone is of little use if one does not know the present position, the destination, headings et cetera. Adding such extra information, such as cost-effectiveness, access and availability, timing, interest of other stakeholders, to the baseline evidence allows creating clinical guidelines.
Before discussing guidelines in cartilage repair, both the need for and the process of formulating and supporting clinical guidelines are worthwhile critical appraisal. Not all guidelines are created equal and not all guidelines are being employed within the scope of the intended use. A general understanding of guideline development processes, including pitfalls and shortcomings, is a valuable tool for present day clinicians.
Guidelines for cartilage repair have been brought forward by scientific societies, public bodies, and insurance companies. These guidelines arrive at a general consensus that cartilage repair, particularly autologous chondrocyte implantation ACI over mosaicplasty and/or microfracture, is an effective and cost-effective treatment option for a defined population. Shortcomings exist: Treatment effectiveness outside of this narrowly defined population is not commented on. Estimates on cost and utility are presented, but obviously highly dependent on geographic location. The dissemination of guidelines is incomplete. The extent to which these guidelines influence health care policy remains unknown.
In summary, guideslines are valuable tools for clinical decision making and to direct clinicians in choosing cartilage repair options. However, their applicability is limited to the scope of the studied populations and dependent on quality and consistency of the primary data. Deduction and extrapolations beyond this scope are not warranted, but research in uncharted new techniques is.
3.3.1 - Progenitor Cells and Ageing
3.3.2 - Osteoarthritis & Ageing
Abstract
Introduction
“Wear and tear”, structural changes, “inflammaging” as well as thinning of cartilage has been implemented as a reason for cartilage degeneration in aging individuals1-3, but not necessarily always associated with osteoarthritis4. While there is plenty of literature dedicated to osteoarthritis, publications dealing with aging of cartilage are relatively scarce and often from the seventies and eighties.Content
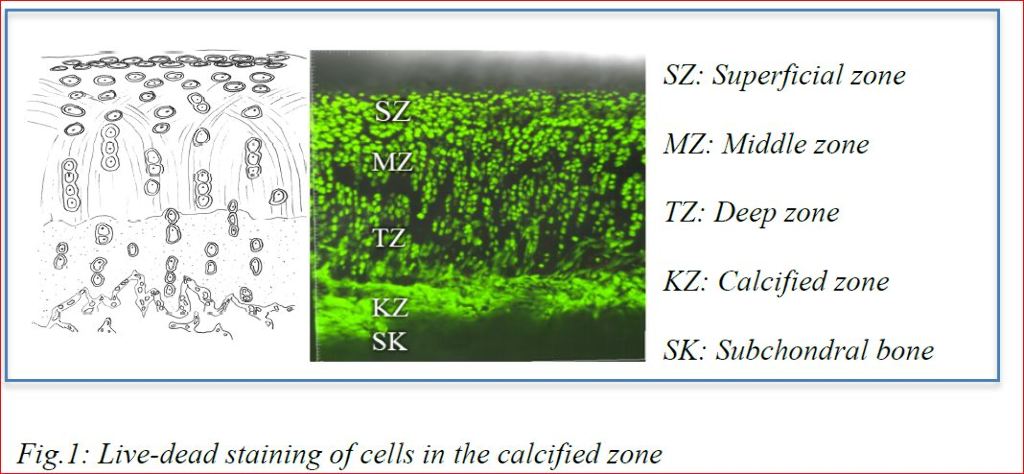
 Structural changes in joint congruency were described, such that over time, joint surfaces and congruency change and adapt including thinning of the cartilage surface. Cartilage degeneration and thinning were attributed to a decreasing anabolic response of chondrocytes to damaging stimuli by increasing their matrix synthesis4. Also age dependent decreased lacunar numbers and hypermineralization of osteocyte lacunae (micropetrosis) were found in aging individuals1 as well as senescence of resident cells or changes in methylation in cartilage and subchondral bone cells with shortening of telomers and changes in epigenetics were recorded1,3,5,6.
Structural changes in joint congruency were described, such that over time, joint surfaces and congruency change and adapt including thinning of the cartilage surface. Cartilage degeneration and thinning were attributed to a decreasing anabolic response of chondrocytes to damaging stimuli by increasing their matrix synthesis4. Also age dependent decreased lacunar numbers and hypermineralization of osteocyte lacunae (micropetrosis) were found in aging individuals1 as well as senescence of resident cells or changes in methylation in cartilage and subchondral bone cells with shortening of telomers and changes in epigenetics were recorded1,3,5,6.A close relationship between subchondral bone and overlying hyaline cartilage is outlined in most of these publications, although still not really understood. One of the reasons, why this is the case and these pathways have not been pursued may be the common belief that the calcified cartilage and tidemark separate the hyaline cartilage from the subchondral bone and no connections between these unities exist in the adult individual and, furthermore, that cells within the calcified cartilage zone are compared to hypertrophic chondrocytes in the growth plate. This goes along with the still held belief that in contrast to bone, there is no permanent remodeling of the hyaline cartilage during the adult life time and that chondrocytes only remodel their immediate environment.
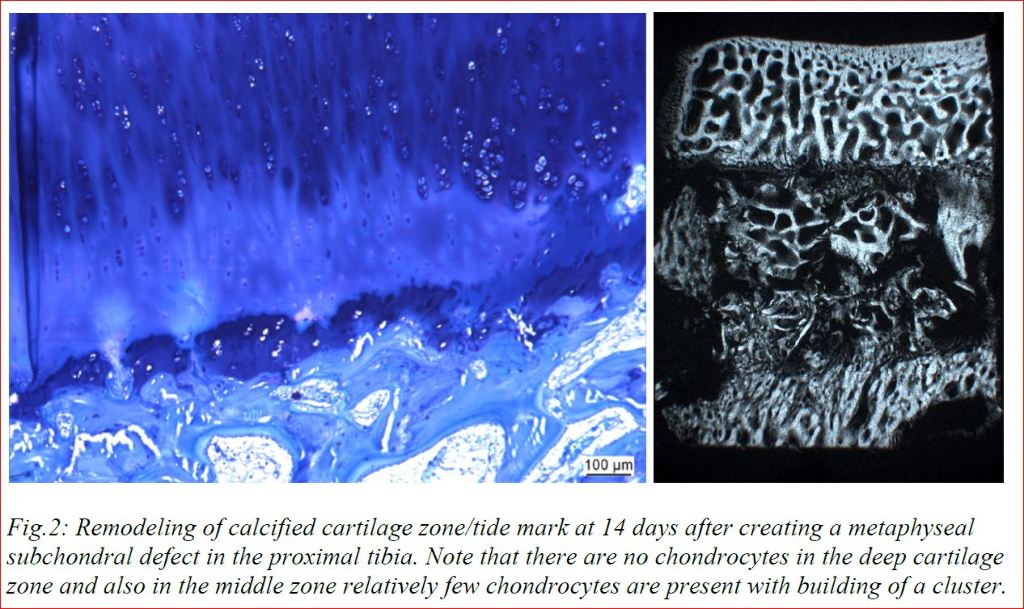
Our research with cartilage samples in vitro (Fig.1) and animal experiments in vivo (Fig.2) show that these views need to be revised. Cells in the calcified cartilage zone were viable and not hypertrophic (or even apoptotic). In addition, in vivo results showed that surgical removal of subchondral bone leads to immediate depletion of chondrocytes in the deep cartilage zone and cartilage degeneration, even if autografts were used to fill the bone defect in vicinity of the hyaline cartilage. Cartilage degeneration could be influenced by treatments addressing proliferation and healing capacity in the subchondral bone. These results lead us to the conclusion that only understanding the tight connection between subchondral bone and hyaline cartilage including the physiological remodeling of healthy cartilage will make it possible to influence the life span of joints without osteoarthritis.
References
1. Loeser RF. Aging processes and the development of osteoarthritis. Curr Opin Rheumatol 2013;25(1):108-13. DOI: 10.1097/BOR.0b013e32835a9428.
2. Bullough PG. The role of joint architecture in the etiology of arthritis. Osteoarthritis Cartilage 2004;12 Suppl A:S2-9. DOI: 10.1016/j.joca.2003.09.010.
3. Hugle T, Geurts J, Nuesch C, Muller-Gerbl M, Valderrabano V. Aging and osteoarthritis: an inevitable encounter? J Aging Res 2012;2012:950192. DOI: 10.1155/2012/950192.
4. Martin JA, Buckwalter JA. Roles of Articular Cartilage Aging and Chondrocyte Senescence in Ostoarthritis. The Iowa Orthopedic Journal 2001;21:1-6.
5. Pippenger BE, Duhr R, Muraro MG, Pagenstert GI, Hugle T, Geurts J. Multicolor flow cytometry-based cellular phenotyping identifies osteoprogenitors and inflammatory cells in the osteoarthritic subchondral bone marrow compartment. Osteoarthritis Cartilage 2015;23(11):1865-9. DOI: 10.1016/j.joca.2015.07.021.
6. Collins JA, Diekman BO, Loeser RF. Targeting aging for disease modification in osteoarthritis. Curr Opin Rheumatol 2018;30(1):101-107. DOI: 10.1097/BOR.0000000000000456.
3.3.3 - Biomarkers of Osteoarthritis and Implications for Therapy (Pre-Recorded)
Abstract
Introduction
Osteoarthritis (OA) is a destructive joint disease with high prevalence, representing a severe health burden and tremendous economic impact. Although cartilage destruction is the hallmark of OA, it is now appreciated that it is a whole joint disease involving changes in all joint tissues including articular cartilage, calcified cartilage, subchondral cortical and trabecular bone, joint capsule, ligaments, tendons, menisci, and the synovium (1,2). Any of these tissues may give rise to biomarkers as a consequence of joint disease, which may be detected in the synovial fluid, blood, and urine. The initiation of the disease can be attributed to direct injury and mechanical disruption of cartilage and other joint tissues, but the progressive changes are dependent on active cell-mediated processes that occur during the time course of the disease. Recent clinical observations and experimental studies have identified OA phenotypes that exhibit common profiles of symptoms, structural abnormalities, and pathophysiological pathways acting independently or in combination within a background of genetic and epigenetic factors (3,4). Multiple risk factors, including injury, aging, obesity, other metabolic disorders, and inflammation, impact on these OA phenotypes and may give insight into strategies for assigning biomarker panels that will stratify subsets of OA with distinct patterns of initiating factors and time courses of disease progression, but with similar end-stage pathology (5). Current therapies involve symptomatic relief of pain, physiotherapy, and ultimately total joint replacement after joint failure. The challenge in the search for disease modifying OA drugs (DMOADs) is that severe cartilage damage, as a critical determinant in OA progression, may not occur or may not be detected radiologically until months or years following the initiating event. Recent progress in identifying and validating biomarkers in at risk patients with early-stage disease holds great promise for impacting on the development of therapeutic approaches and the design of clinical trials.
Content
Efforts in the OA field have been focused on developing a biomarkers toolbox, based on experimental findings to enable understanding of mechanisms of pathogenesis and how they might be translated to therapy. Biomarkers are defined as surrogates for clinical endpoints that, in the case of OA, would substitute for outcomes such as pain, functional status, or candidacy for total joint replacement (6,7). While the importance of molecular biomarkers is increasingly recognized, no OA-related biomarker has been adopted for routine use in clinical practice, thus reflecting the lack of pharmacological structure-modifying DMOADs. Insights into the pathologic and pathogenic processes associated with the initiation and progression of OA have been provided by remarkable advances in defining the composition and structural organization of articular cartilage and in elucidating the molecular mechanisms regulating the anabolic and catabolic activities of chondrocytes. Much of the search for biomarkers, therefore, has focused on adult articular cartilage, which is a complex tissue of matrix proteins with a resident population of chondrocytes that are normally quiescent and maintain the matrix in a low-turnover state. Until recently, chondrocyte phenotypes were defined according to their developmental stage, for example, as proliferating, pre-hypertophic, and hypertrophic chondrocytes, or state of maturity, i.e., articular, and fibrocartilage chondrocytes. During OA, articular chondrocytes undergo phenotypic modulation, promoting matrix destruction, abnormal attempts at repair, and apoptotic cell death. Chondrocytes in aging cartilage, particularly those in the superficial zone, become senescent, possibly as a homeostatic protective mechanism, and release proteins involved in their senescence associated secretory phenotype (SASP). There are many inflammatory markers associated with SASP, which become more prominent when OA disease is present. Of the many cartilage biomarkers under investigation, the most consistent are related to matrix degradation such as the neo-epitopes created by enzymatic cleavage of collagen, aggrecan, and COMP (6). These OA-related events may be regulated by inflammatory mediators deriving from the synovitis in the joint, but also by the low-grade inflammation in the cartilage itself. While synovium is known to be heterogeneous with many cell populations such as fibroblasts, macrophages, and immune cells, recent evidence by single-cell RNAseq showed 12 distinct OA synovial cell phenotypes (9). In contrast, the dogma that cartilage contains one dominant cell type has been upended by RNAseq analyses showing 7 distinct OA chondrocyte phenotypes, particularly during OA progression (9, 10). The study of Chou et al. (9) also predicted the upstream mediators associated with these phenotypes in either synovial tissue or cartilage, supporting the concept that cross talk between synovial cells and cartilage plays critical roles in OA.
Similarly, there have been significant advances in characterizing the structural and functional alterations in periarticular bone in OA and in defining the factors and processes that control the remodeling and adaptation of skeletal tissues to local mechanical and systemic factors (2). Thus, products of bone resorption and formation have been added to the OA biomarker toolbox (6). Since the responses of bone cells to biomechanical damage in early stages of OA may be involved in cross talk with cartilage to initiate and promote damage, it will be important to include bone biomarkers in the toolbox.
Pre-clinical studies in vitro and in vivo have led to the development and testing of therapeutic agents and treatment interventions that have the potential to slow or attenuate OA progression, but significant challenges exist in translating them for clinical use. Advances in the development of advanced MRI technologies and biomarker assays that reflect changes in the synthetic and degradation products of the cartilage matrix and bone hold promise for early diagnosis, for assessing disease progression, and for more accurately monitoring of the efficacy of disease-modifying therapies; these, however, need further validation to be of clinical utility. Currently available pharmacological therapies address pain, which remains a major cause of disability in individuals with OA and further studies are needed to define the sources and mechanisms involved in the pathogenesis of joint pain and associated pain biomarkers to assess individual patients. Therefore, there is a great need for defining sets of biomarkers that will identify individuals who are at risk at early stages for more rapidly progressing to OA with the goal of developing structure modifying drugs that also ameliorate pain. At the very least, the biomarker subsets could be used to define cohorts of OA patients with different etiologies and risk factors to enable the design of more rational clinical trials for successful translation of phenotype-specific targeted therapies. The availability of appropriate biomarkers could enhance our ability to evaluate all stages and subsets of OA with regard to diagnosis, prognosis, drug development, and treatment. Overall, the validation of biomarkers as tools for monitoring disease status and patient responses to therapy is critical for facilitating drug development for OA.
References
1. Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis and rheumatism 2012;64:1697-1707.
2. Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol 2016.
3. Goldring MB, Berenbaum F. Emerging targets in osteoarthritis therapy. Curr Opin Pharmacol 2015;22:51-63.
4. Singh P, Marcu KB, Goldring MB, Otero M. Phenotypic instability of chondrocytes in osteoarthritis: on a path to hypertrophy. Ann N Y Acad Sci 2019;1442:17-34.
5. Favero M, Ramonda R, Goldring MB, Goldring SR, Punzi L. Early knee osteoarthritis. RMD Open 2015;1:e000062.
6. Kraus VB, Karsdal MA. Foundations of osteoarthritis section 3: clinical monitoring in OA chapter 15: biomarkers. Osteoarthritis Cartilage 2021.
7. Bay-Jensen AC, Mobasheri A, Thudium CS, Kraus VB, Karsdal MA. Blood and urine biomarkers in osteoarthritis - an update on cartilage associated type II collagen and aggrecan markers. Curr Opin Rheumatol 2022;34:54-60.
8. Favero M, Belluzzi E, Trisolino G, Goldring MB, Goldring SR, Cigolotti A, Pozzuoli A, Ruggieri P, Ramonda R, Grigolo B, Punzi L, Olivotto E. Inflammatory molecules produced by meniscus and synovium in early and end-stage osteoarthritis: a coculture study. J Cell Physiol 2019;234:11176-11187.
9. Chou CH, Jain V, Gibson J, Attarian DE, Haraden CA, Yohn CB, Laberge RM, Gregory S, Kraus VB. Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Sci Rep 2020;10:10868.
10. Ji Q, Zheng Y, Zhang G, Hu Y, Fan X, Hou Y, Wen L, Li L, Xu Y, Wang Y, Tang F. Single-cell RNA-seq analysis reveals the progression of human osteoarthritis. Ann Rheum Dis 2019;78:100-110.
Acknowledgments
I am grateful for research grant support during the later stage of my career and ecellent collaborations:
1. NIH-R01-AG022021: “ESE1 a novel transcriptional regulator of cartilage remodeling”
• Miguel Otero (Hospital for Special Surgery)
2. NIH-RC4 AR060546: “Defining Common Molecular Parameters for Onset and Progression of Osteoarthritis”
• Kenneth B. Marcu (Stony Brook University)
• Yefu Li (Harvard Medical School)
• Roland Wolkowicz (San Diego State Univ)
3. NIH-R21 AR054887: “Epigenetic Regulation of MMP-13”
• Richard Oreffo (Trudy Roach), et al. (Univ of Southampton)
• Ko Hashimoto (Tohoku Univ, Sendai, Japan)
4. Marjolein van der Meulen, PI (Cornell Univ): R21-AR064034: “Role of cartilage matrix in a model of load-induced OA”
5. Carl Blobel, PI (HSS): Arthritis Foundation IRG-6353: “Role of iRhom2 and ADAM17 in osteoarthritis”



