Presenter of 1 Presentation
RAPID CONVALESCENT PLASMA STERILIZATION USING HIGH DOSE RATE ELECTRON RADIATION
Abstract
Background and Aims
Passive antibody administration through convalescent plasma has shown benefit in treating COVID-19 in the early stages of the disease in patients >65 years old, and in other viral outbreaks. A practical, rapid method to sterilize convalescent plasma while also maintaining antibody function would be valuable for safe treatment in future viral pandemics. Plasma sterilization by irradiation requires kGy of dose to deactivate bacteria and viruses of concern. Conventional lab-based irradiators would require days to reach such doses, while ultra-high dose rate irradiation (FLASH) would require minutes. We present a proof-of-concept on sterilizing plasma with 25 kGy in approximately 3 minutes without damaging the antibodies in the plasma.
Methods
A Varian Trilogy LINAC was configured for 16 MeV FLASH electron irradiation. Frozen aliquots of convalescent plasma from patients with COVID-19 were placed in a 3D printed holder submerged in liquid aiming to preserve sample temperature (RT, 4C or -20C). The number of pulses was estimated with EBT-XD film. Samples were irradiated with a dose of 25 kGy in ~33,330 pulses over 185 seconds. Antibody binding against the receptor-binding domain (RBD) of the S1 region of SARS-CoV-2 was measured by ELISA pre- and post-irradiation.
Results
Frozen plasma aliquots from 10 COVID-19 convalescent plasma donors were irradiated in frozen state to 25 kGy dose. IgG antibody binding against SARS-CoV-2 RBD after irradiation remained at 90.8% of non-irradiated samples (Figure; OD 1.25 vs. 1.36, p< 0.0003).
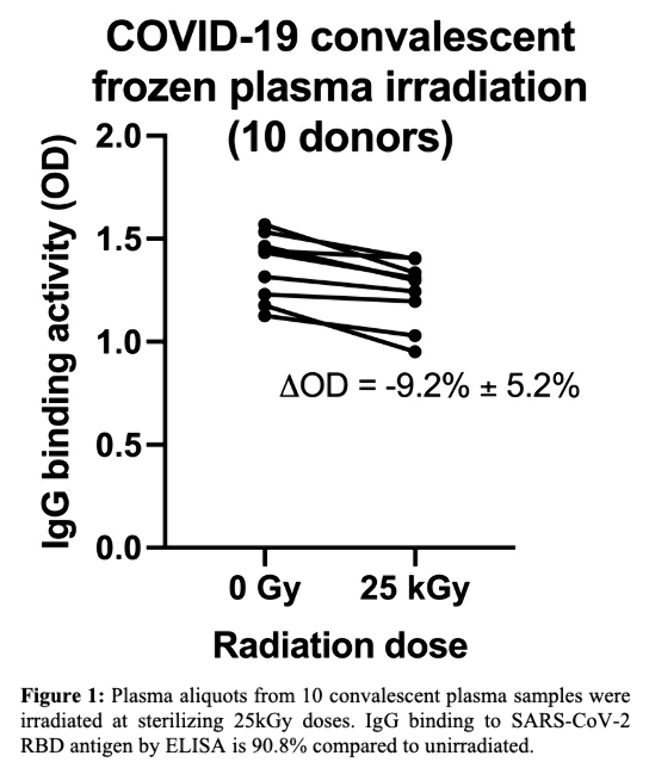
Conclusions
FLASH irradiation allows for rapid sterilization of blood plasma from potential pathogens while largely preserving antibody binding function and specificity.
Author Of 2 Presentations
RAPID CONVALESCENT PLASMA STERILIZATION USING HIGH DOSE RATE ELECTRON RADIATION
Abstract
Background and Aims
Passive antibody administration through convalescent plasma has shown benefit in treating COVID-19 in the early stages of the disease in patients >65 years old, and in other viral outbreaks. A practical, rapid method to sterilize convalescent plasma while also maintaining antibody function would be valuable for safe treatment in future viral pandemics. Plasma sterilization by irradiation requires kGy of dose to deactivate bacteria and viruses of concern. Conventional lab-based irradiators would require days to reach such doses, while ultra-high dose rate irradiation (FLASH) would require minutes. We present a proof-of-concept on sterilizing plasma with 25 kGy in approximately 3 minutes without damaging the antibodies in the plasma.
Methods
A Varian Trilogy LINAC was configured for 16 MeV FLASH electron irradiation. Frozen aliquots of convalescent plasma from patients with COVID-19 were placed in a 3D printed holder submerged in liquid aiming to preserve sample temperature (RT, 4C or -20C). The number of pulses was estimated with EBT-XD film. Samples were irradiated with a dose of 25 kGy in ~33,330 pulses over 185 seconds. Antibody binding against the receptor-binding domain (RBD) of the S1 region of SARS-CoV-2 was measured by ELISA pre- and post-irradiation.
Results
Frozen plasma aliquots from 10 COVID-19 convalescent plasma donors were irradiated in frozen state to 25 kGy dose. IgG antibody binding against SARS-CoV-2 RBD after irradiation remained at 90.8% of non-irradiated samples (Figure; OD 1.25 vs. 1.36, p< 0.0003).

Conclusions
FLASH irradiation allows for rapid sterilization of blood plasma from potential pathogens while largely preserving antibody binding function and specificity.
REAL-TIME OPTICAL OXIMETRY UNDER IRRADIATION
Abstract
Background and Aims
Transient changes in oxygen tension in tissues taking place during FLASH radiotherapy may explain its biological effects. However, because the kinetics of oxygen depletion and recovery occur on a very short timescale, it is challenging to measure these effects in vivo using existing methods. Here we developed a real-time optical oximetry system with millisecond temporal resolution to elucidate early radiochemistry under irradiation.
Methods
Oxygen measurements were performed in vitro using the phosphorescence quenching method and a water-soluble molecular nanoprobe (Fig. 1). An epifluorescence fiber-coupled system was designed and built. The system was validated using a standard dissolved oxygen meter. The changes in oxygen per unit dose (G-value) were quantified in response to irradiation by 320 kVp x-ray and 16 MeV electron beam at dose rates ranging from 0.04 Gy/s to 100 Gy/s.
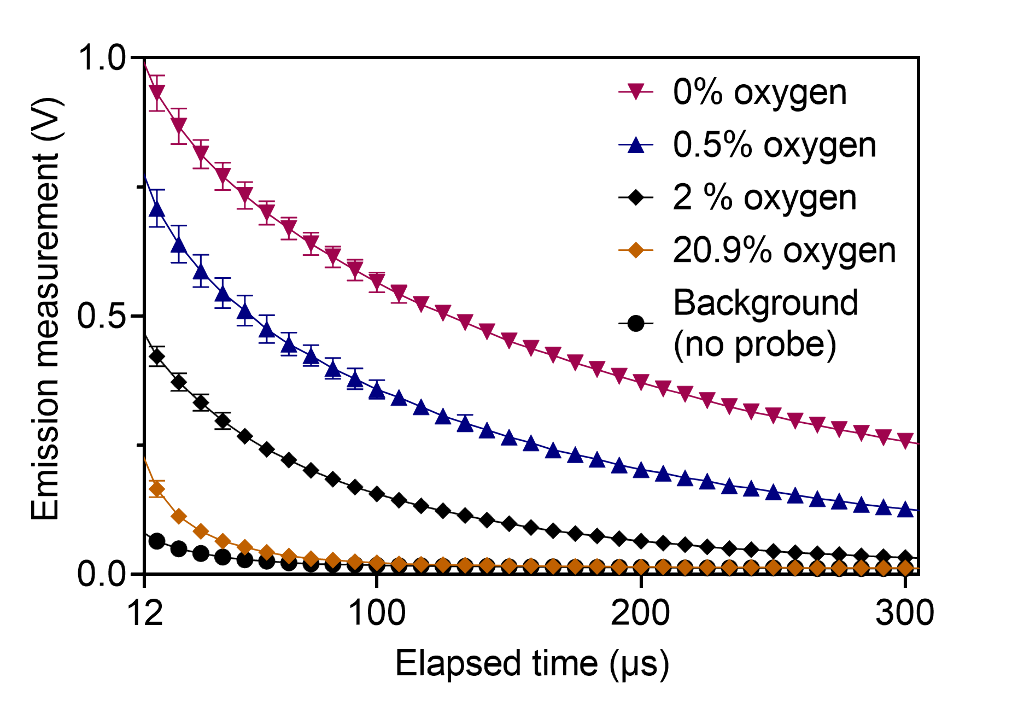
Results
Transient oxygen depletion of phosphate buffer solution under standard kV X-ray irradiation was continuously measured every millisecond (Fig. 2). The samples at normoxia with oxygen concentration of 150–240 µM had constant G-value of 0.54 uM/Gy, however hypoxic samples (15 µM and below) had significantly lower G-values (Fig. 3).
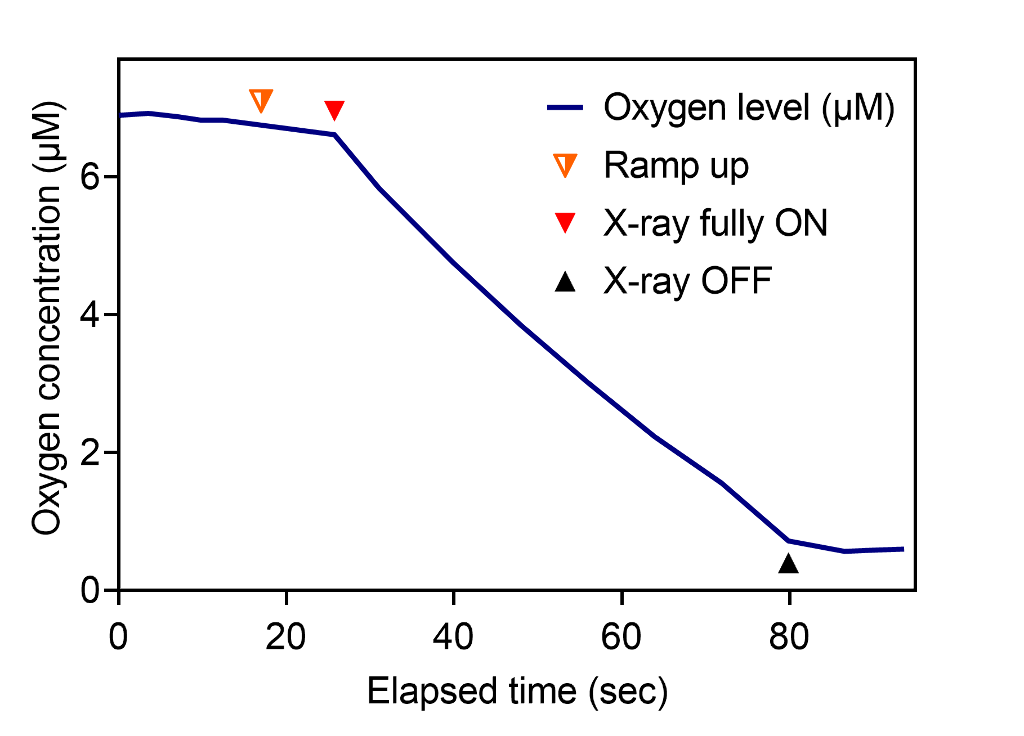
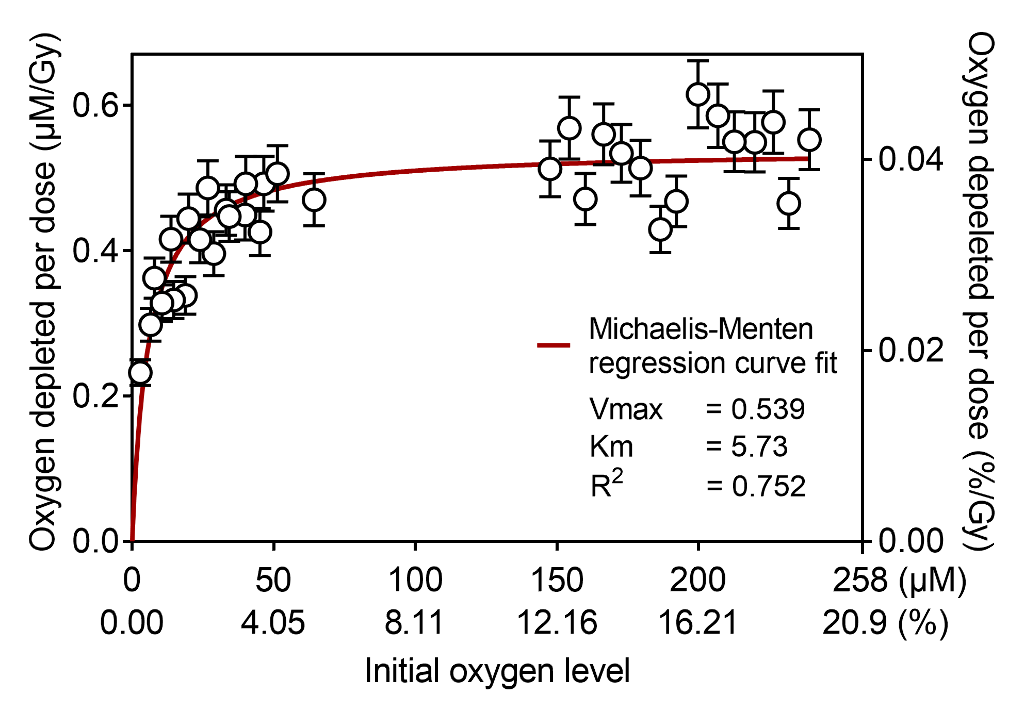
Conclusions
Our observations suggest that oxygen depletion rate decreases under hypoxia, with the measured data being a good fit to Michaelis-Menten kinetics. Future works will measure oxygen depletion kinetics of biomimetic lipid emulsions and in vivo mice under irradiation. We anticipate that the proposed method will capture crucial millisecond-order oxygen change after individual e-beam pulses.

