The Conference will officially run on Central European Time (CET).
To convert to your local time click here.
The viewing of sessions cannot be accessed from this conference calendar.
All sessions are accessible via the Main Lobby on the Virtual Platform.
Thu, 01.01.1970
A RANDOMIZED DOSE ESCALATION STUDY OF FLASH VS STANDARD DOSE RATE PROTON RADIOTHERAPY FOR CANINE OSTEOSARCOMA
Abstract
Background and Aims
To examine the impact of ultra-high dose rate, “FLASH,” proton RT (F-PRT) vs standard dose rate PRT (S-PRT), we have performed a randomized dose escalation study of F-PRT vs S-PRT for dogs with osteosarcoma (OSA). Standard of care treatment typically involves complete or partial amputation, allowing for pathological and molecular examination of tumor vs normal tissue PRT effects.
Methods
Following owner signing informed consent, dogs were randomly assigned to F-PRT vs S-PRT at escalating doses from 4-24 Gy. Under fluoroscopic guidance, fields were designed to treat segments of tumor vs normal metaphyseal bone. Five days after PRT, dogs underwent limb amputation and samples of tumor, normal bone, skin and subcutaneous soft tissues were collected from irradiated and unirradiated sites.
Results
To date 27 dogs have completed treatment, with 4 remaining dogs to be treated in the 24 Gy cohort. Acute toxicity following PRT has been observed in 2 dogs, with one dog having a pathologic fracture and one dog experiencing intertumoral hemorrhage following treatment with 12 Gy S-PRT and F-PRT, respectively. Preliminary histologic analyses suggest no difference in tumor necrosis between F-PRT and S-PRT and molecular analysis demonstrates F-PRT dramatically reduces induction of TGF-beta signaling in normal tissues as compared to S-PRT. More detailed molecular studies using RNAseq are ongoing to help define mechanisms of F-PRT effects.
Conclusions
We have performed the first study of F-PRT in dogs with spontaneous OSA demonstrating both the clinical feasibility and potential utility of this novel modality in a genetically heterogeneous model system.
RAPID CONVALESCENT PLASMA STERILIZATION USING HIGH DOSE RATE ELECTRON RADIATION
Abstract
Background and Aims
Passive antibody administration through convalescent plasma has shown benefit in treating COVID-19 in the early stages of the disease in patients >65 years old, and in other viral outbreaks. A practical, rapid method to sterilize convalescent plasma while also maintaining antibody function would be valuable for safe treatment in future viral pandemics. Plasma sterilization by irradiation requires kGy of dose to deactivate bacteria and viruses of concern. Conventional lab-based irradiators would require days to reach such doses, while ultra-high dose rate irradiation (FLASH) would require minutes. We present a proof-of-concept on sterilizing plasma with 25 kGy in approximately 3 minutes without damaging the antibodies in the plasma.
Methods
A Varian Trilogy LINAC was configured for 16 MeV FLASH electron irradiation. Frozen aliquots of convalescent plasma from patients with COVID-19 were placed in a 3D printed holder submerged in liquid aiming to preserve sample temperature (RT, 4C or -20C). The number of pulses was estimated with EBT-XD film. Samples were irradiated with a dose of 25 kGy in ~33,330 pulses over 185 seconds. Antibody binding against the receptor-binding domain (RBD) of the S1 region of SARS-CoV-2 was measured by ELISA pre- and post-irradiation.
Results
Frozen plasma aliquots from 10 COVID-19 convalescent plasma donors were irradiated in frozen state to 25 kGy dose. IgG antibody binding against SARS-CoV-2 RBD after irradiation remained at 90.8% of non-irradiated samples (Figure; OD 1.25 vs. 1.36, p< 0.0003).
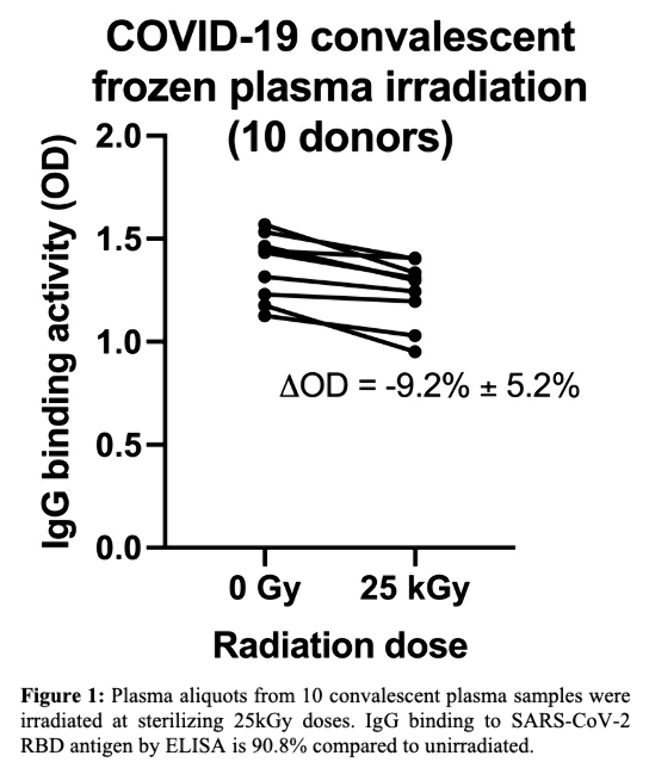
Conclusions
FLASH irradiation allows for rapid sterilization of blood plasma from potential pathogens while largely preserving antibody binding function and specificity.
CREATING CLINICALLY COMPETITIVE FLASH PROTON THERAPY TREATMENT PLANS
Abstract
Background and Aims
FLASH proton therapy (FLASH-PT) is beginning the transition into clinical practice. The beam delivery, dose rates, and fractionation schemes for FLASH-PT differ from standard radiotherapy. Hence, no guidelines exist regarding the development of treatment plans for this novel technique. This study aims to determine if FLASH-PT treatment plans can be developed and if the in silico results are comparable to the treatment plans produced for standard radiotherapy.
Methods
FLASH-PT and IMPT treatment plans were created using a novel research version of the MIROpt TPS, developed by Ion Beam Applications SA from the open source version of UCLouvain, for nine patient cases of bone (3), brain (3), and lung metastases (3), previously clinically treated with 3DCRT or VMAT. A FLASH proton dose rate of ≥ 40 Gy/s was included as an optimisation criterion for each patient case. Treatment plans were compared using dose volume histograms (DVHs), boxplots, and the Wilcoxon Rank Sum Test with a 5% significance level and using DVH parameters V100%, V95%, V50%, D99%, D95%, and D2% for target and body structures.
Results
No significant differences were found between the optimised FLASH-PT plans and the clinical 3DCRT/VMAT and optimised IMPT plans.
Conclusions
The FLASH-PT treatment plans created in this study produced in silico results comparable to those of clinically competitive treatment plans. Future work involves the verification of the calculated dose against delivered dose and dose rate, to ensure that the produced treatment plans can be delivered safely and accurately, confirming the feasibility of the clinical implementation of conformal FLASH-PT.
HYPOFRACTIONATED FLASH RADIOTHERAPY VERSUS CONVENTIONAL RADIOTHERAPY IN AN IMMUNOCOMPETENT RAT GLIOMA MODEL
Abstract
Background and Aims
For a clinical translation of FLASH radiotherapy, evidence of an enhanced therapeutic index within the same biological system is needed. In this study we aim to simultaneously investigate the tumor response (TCP) and skin toxicity (NTCP) of hypofractionated FLASH compared to conventional radiotherapy (CONV) in a fully immunocompetent rat glioma model.
Methods
Fisher 344 rats with subcutaneously inoculated NS1 glioma cells were treated with FLASH (450-550 Gy/s) or CONV in three fractions of either 8 Gy, 12.5 Gy or 15 Gy (n=9-10) using a 10 MeV electron beam. Animals were followed for 100 days. The tumor control probability was determined as the ratio of animals with no tumor progression at the end of the study period. Local dermal side effects were evaluated weekly.
Results
There was a statistically significant difference in overall survival between controls and all treatment groups, but no significant difference between FLASH and CONV for any of the dose levels (log-rank test). The tumor control probability for 3x8 Gy, 3x12.5 Gy and 3x15 Gy, respectively, were 3/9, 5/10 and 9/10 for CONV and 3/10, 5/9 and 9/10 for FLASH. Local dermal side effects were generally mild, consisting of hair loss, erythema, and dry desquamation. The ratio of animals with erythema or more severe effects during the study period was 78%/60%/90% for CONV and 50%/78%/70% for FLASH.
Conclusions
In this study we demonstrate that hypofractionated FLASH is equally effective as CONV in terms of controlling glioma in rats. No difference in acute effects could be resolved. Late effects will be histologically evaluated.
DEMONSTRATION OF THE FLASH EFFECT USING MERGED FIELDS IN PROTONS AT THE BRAGG PEAK
Abstract
Background and Aims
One barrier to clinical implimentation of FLASH is the challenge of treating large clinical volumes at FLASH dose rates. The aim of this work is demonstrating the biological feasibility of delivering multiple volumes, each at FLASH dose rates, forming larger combined volumes, maintaining the FLASH effect globally even if the total treatment time exceeds a FLASH time scale.
Methods
Similar to previous work[1], proton radiation was delivered to the abdomens of 15 healthy mice using a 2 cm spread-out Bragg peak (SOBP). Treatment areas were irradiated to 15 Gy, with 9 mice receiving their dose at FLASH dose rates (100 Gy/s) and 6 mice at conventional dose rates (0.1 Gy/s). The mice received two adjacent fields, each an 11 mm-diameter circle, with the mouse repositioned between each delivery. Normal tissue damage was assessed by the EdU staining method[2], evaluating uptake in irradiated intestinal crypt cells.
[1] Int J Part Ther 2021; doi: https://doi.org/10.14338/IJPT-20-00095
[2] Int J Radiat Oncol Biol Phys . 2020 Feb 1;106(2):440-448. doi: 10.1016/j.ijrobp.2019.10.049.
Results
Separation was observed between FLASH and conventional mice in the number of regenerated cells per crypt (Figure 1) demonstrating that FLASH radiotherapy spares normal intestinal tissue at 3.5 days post 15 Gy SOBP radiation in comparison to standard proton radiotherapy.

Conclusions
These results demonstrate the feasilibity of “merged-field” FLASH. This efficient technique will allow for large, clinical volumes to be treated by combining smaller Bragg peak IMPT FLASH treatments and simultaneously maintaining the normal-tissue sparing benefits of the FLASH effect across the whole volume.
A RANDOMIZED CLINICAL PHASE-III-TRIAL COMPARING SINGLE-HIGH DOSE FLASH-RADIOTHERAPY VERSUS CONVENTIONALLY FRACTIONATED RADIOTHERAPY IN CAT-PATIENTS WITH SQUAMOUS CELL CARCINOMA: EARLY STOPPING DUE TO LATE TOXICITY
Abstract
Background and Aims
Normal tissue-sparing property of FLASH-RT has been shown in various studies, including a dose-escalating trial with single-dose FLASH-RT (25-41Gy) in cat-patients. Results prompted us to design this prospective, randomized clinical phase-III-trial in cat-patients with spontaneous tumors, to compare single-high-dose FLASH-RT to a standard of care (SOC); with tumour control-rate at 1 year as primary endpoint (hypothesis= 95% with FLASH-RT versus 71% for SOC, alpha=0.05 and beta=0.2, 29 cats needed).
Methods
Ethic’s approval was obtained (ZH204/18) and cats with T1-T2 N0 carcinomas of the nasal planum were randomly assigned to 2 arms of electron radiation. Arm 1 used 10x4.8Gy (90%IDL), delivered in one week with a 6MeV linear accelerator, dose rate of 600MU/min. Arm 2 used 1x30Gy (89%IDL) with eRT6/Oriatron, delivered in 20ms using 3 pulses, instantaneous dose rate of 6.3x106Gy/s (mean dose rate 1700Gy/s).
Results
While acute side effects were mild to moderate and similar in both arms, the trial was prematurely stopped due an excess of maxillary bone necrosis which occurred 9-12 months after RT in 3/7 cats treated with FLASH-RT (43%), as compared to 0/9 cats in SOC. Regarding the primary endpoint, all cats were free of tumor progression at 1 year in both arms, but one tumor progression occurred later in FLASH-RT arm. Overall survival rates were similar in both arms, 690 days for SOC and 680 days for FLASH.
Conclusions
When compared to SOC, 1x30Gy-FLASH was beyond the maximal tolerated dose, causing severe late toxicity without better tumor control.
Acknowledgments: Krebsliga, KFS-4438-02-2018: Phase III clinical trial on cat patients

