Welcome to the e-INS 2023 Interactive Program
REAL-WORLD OUTCOMES USING DBS SYSTEMS WITH DIRECTIONALITY AND MULTIPLE INDEPENDENT CURRENT CONTROL: EXPERIENCE IN THE USA (ID 34)
Abstract
Introduction
Deep Brain Stimulation (DBS) has been substantiated by several randomized controlled trials as an effective strategy for reducing the motor complications in Parkinson's disease (PD).1-3 This motor improvement has been shown in cohorts to possibly be sustained for up to 10 years.4 Clinical data collected from a wide variety of implanting centers using local standard of care has revealed overall improvements in PD disease symptoms and quality-of-life when applying DBS therapy. Here, we present preliminary outcomes from an ongoing, prospective, multicenter study conducted in the United States of patients implanted with directional DBS Systems capable of multiple independent current control (MICC) for use in the management of the motor signs and symptoms of levodopa- responsive PD.
Materials / Methods
Prospectively-enrolled participants were implanted with a DBS system (VerciseTM, Boston Scientific, Valencia, CA, USA), a multiple-source, constant- current device, and were assessed up to 3-years post-implantation. Clinical measures recorded at baseline and during study follow-up included: MDS-Unified Parkinson's disease Rating Scale (MDS-UPDRS), Parkinson's Disease Questionnaire (PDQ-39), Global Impression of Change (GIC), and Non-Motor Symptom Assessment Scale (NMSS), and adverse events.
Results
A total of 111-patients (mean age: 64.1 ± 8.7 years, 73% male, disease duration 9.7 ± 5.3 years, n = 108) have been enrolled to date, and 93 have devices which have been activated. A 56.4% improvement (28.2-points, p<0.0001) in motor function was noted at 6-months as assessed by MDS-UPDRS III in the meds "off" stimulation “on” condition. Quality of life was improved with an 8.4-point change in the PDQ-39 Summary Index (p<0.0001). This change exceeded the minimal clinically important difference (MCID) for PDQ-39 which is 4.7-points.5 At 6-months post-DBS, a categorical subject measure revealed that 98% of patients and 95% of clinicians reported improvements (GIC). There have been no lead breakages.
Discussion
In this study, collection of real-world data across multiple implanting centers is informing on longer-term outcomes of MICC-based DBS systems.
Conclusions
Real-world outcomes from this large, prospective, multicenter outcomes study demonstrate improvement in quality-of-life and motor function following DBS, and overall satisfaction among patients and clinicians. Data from this study will continue to provide insight regarding the application of the MICC-based directional DBS Systems for PD in clinical practice.
References
1. Okun MS, Gallo BV, Mandybur G, et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson's disease: an open-label randomised controlled trial. Lancet Neurol. 2012 Feb;11(2):140-9.
2. Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med. 2013 Feb 14;368(7):610-22.
3. Vitek JL, Jain R, Chen L, Tröster AI, et al. Subthalamic nucleus deep brain stimulation with a multiple independent constant current-controlled device in Parkinson's disease (INTREPID): a multicentre, double-blind, randomised, sham-controlled study. Lancet Neurol. 2020 Jun;19(6):491-501.
4. Deuschl G, Paschen S, Witt K. Clinical outcome of deep brain stimulation for Parkinson's disease. Handb Clin Neurol. 2013;116:107-28.
5. Horváth K, Aschermann Z, Kovács M, et al. Changes in Quality of Life in Parkinson's Disease: How Large Must They Be to Be Relevant? Neuroepidemiology. 2017;48(1-2):1-8.
Learning Objectives
1. To assess real-world motor function outcomes when utilizing MICC-based DBS systems with directionality in patients with Parkinson’s Disease.
2. To assess real-world safety when utilizing MICC-based DBS systems with directionality on patients with Parkinson’s Disease.
3. To assess real-world quality-of-life outcomes when utilizing MICC-based DBS systems with directionality on patients with Parkinson’s Disease.
O023 - COMBINED USE OF BETA-FREQUENCY PEAK MAGNITUDE AND PATIENT-SPECIFIC ANATOMY TO IDENTIFY OPTIMAL STIMULATION PARAMETERS FOR DEEP BRAIN STIMULATION IN THE SUBTHALAMIC NUCLEUS (ID 221)
Abstract
Introduction
Deep brain stimulation (DBS) is an established therapy for Parkinson’s disease (PD). Recent commercial battery technology can simultaneously deliver therapeutic stimulation and record neural data. This pilot study investigates the relationship between two neural parameters from the subthalamic nucleus (STN): 1) beta frequency power, and 2) the volume of neural activation (VNA) restricted to monopolar stimulation, during initial programming aimed at maximizing clinical benefit without stimulation induced adverse effects.
Materials / Methods
PD patients (n = 2; hemispheres = 4) receiving bilateral STN DBS with a battery capable of recording were recruited. During the initial programming visit, local field potential (LFP) recordings were collected in conjunction with standard motor testing. Recordings were used to identify the DBS electrode with the maximum LFP beta peak. Subsequently, stimulation amplitude was gradually increased through beta-identified electrodes to assess the impact of varying the VNA on therapeutic window. The relationships between VNA/STN overlap and stimulation-induced beta suppression and motor symptom improvement were assessed at each amplitude titration step (Figure 1).
Results
Across subjects/hemispheres, we observed that with increased VNA/STN overlap, there was an association between improved rigidity (p<0.01 at >10mm³ overlap) and LFP beta power suppression (p<0.01 at >20mm³overlap). The rate of stimulation induced paresthesia was also significantly correlated with VNA spread outside the STN (R2=0.76).
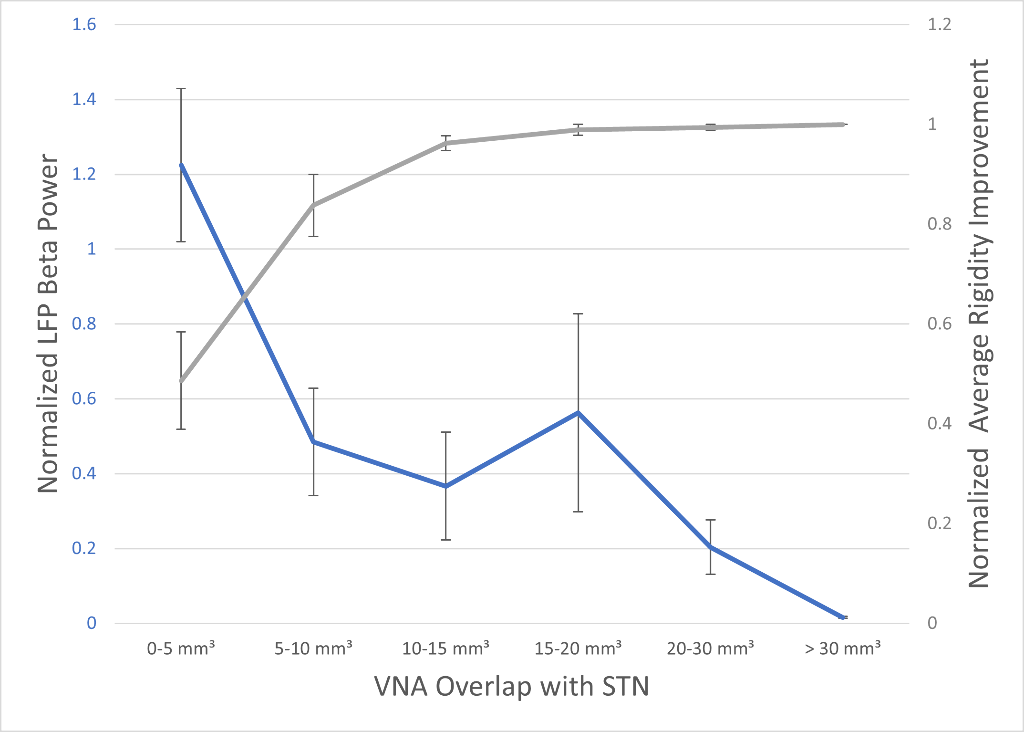
Figure 1. Plot showing the LFP beta power response (blue trace) and improved rigidity response (grey trace) as the VNA/STN overlap increased with increasing stimulation amplitude.
Discussion
Pilot data suggests that the combination of both localization of DBS activity within STN and beta LFP could prospectively inform the therapeutic target and outcome improvement. As stimulation amplitude was titrated up, improvement in rigidity was associated with simultaneous reduction in LFP beta power and increased activation of the anatomical stimulation target. Conversely, higher simulation amplitudes with VNA spread outside of the STN were associated with paresthesia. A better understanding of the relationship between patient-specific therapy, sensing and VNA dynamics across a larger patient cohort may allow for clinically significant improvements in the DBS programming workflow.
Conclusions
Visualization of patient-specific anatomy can be used to demonstrate overlap between LFP signal location and optimal VNA. If this relationship generalizes across a larger cohort, LFP sensing data may provide sufficient information for objective guidance of programming electrode and amplitude.
Learning Objectives
1. Understand the relationship of the VNA with the STN when adjusting stimulation parameters and observe LFP and patient outcome behavior.
2. Understand how the VNA spread outside of the STN impacts the rate of stimulation induced paresthesias
3. Observe how beta-identified electrodes align with the STN anatomy.
O024 - DEVELOPING AN AUGMENTATION APPROACH FOR ITBS IN MAJOR DEPRESSIVE DISORDER USING SYNCHRONISED TACS (ID 106)
Abstract
Introduction
Transcranial magnetic stimulation (TMS) is approved for treating major-depressive-disorder (MDD), a condition that affects 1 in 7 people during their lifetime1. An increasingly popular protocol, due to short session time, is intermittent theta burst stimulation (iTBS), in which TMS pulse triplets repeat at 5Hz (a “theta” frequency)2. Response to TMS, including iTBS, is highly variable3,4. We are examining a relatively inexpensive, easy-to-implement, approach to make iTBS act faster, for more people, by modifying “brain state” at the time of stimulation.
Materials / Methods
One aspect of “brain state” is oscillatory activity, generated by neural-circuit dynamics. Oscillatory activity mediates excitability within brain areas and connectivity between areas5. Pre-treatment fronto-central theta oscillatory power, measured with electroencephalography (EEG), is correlated with response to antidepressant medications6. Li et al.7 found that performance of a theta-eliciting task prior to TMS was associated with better clinical response, and post-task theta predicted response. However, task-based augmentation is unsuitable for people with more severe depression, characterised by cognitive and motivational difficulties8,9.
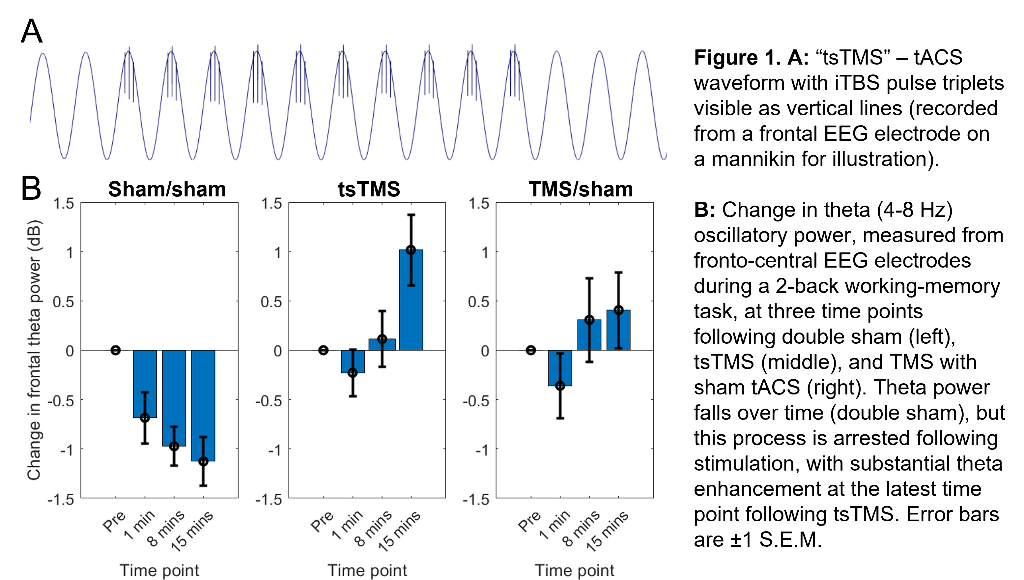
Our approach seeks to enhance theta activity during and after iTBS (and thus augment iTBS efficacy) using concurrent, synchronised, transcranial alternating-current stimulation (tACS). (Building on work showing combined tACS-TMS enhances oscillatory power for traditional “rTMS” protocols10). tACS is delivered at a theta-frequency (5Hz), with 1mA peak-to-peak amplitude, using 9cm2 round-electrodes at F3 (anode) and TP9 (cathode). iTBS at 80% resting-motor-threshold (20 cycles - 600 pulses, 3.3 minutes) is delivered via a 70mm figure-eight coil over F3, with pulse triplets timed to occur at the tACS peaks (Fig.1A).
Results
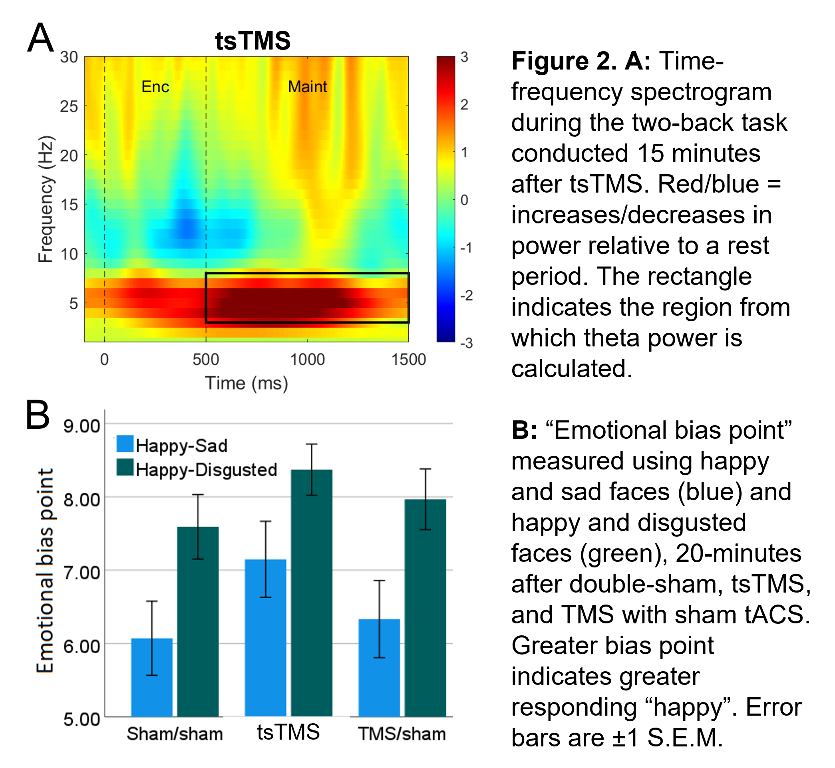
In an ongoing non-patient study (current N=12, 6 female, age±SD 24.1±5.6 years), theta activity during an n-back working-memory task is recorded before, and 1/8/15-minutes after, one session of “tACS-synchronised TMS” (tsTMS), TMS with sham-tACS, or double-sham. In the double-sham condition, theta decreased with repetition of the n-back task. In both stimulation conditions, this reduction was arrested, with an increase in theta (relative to pre-stimulation) at the 8/15-minute time points. At 15-minutes, theta was greatest in the tsTMS condition (Fig.1B,2A). At 20-minutes, positive emotional bias (a marker of potential antidepressant efficacy11,12, obtained from ratings of “happy” or “sad” to briefly-presented facial expressions) was greatest after tsTMS (followed by TMS alone, Fig.2B).
Discussion
The results suggest tACS-synchronisation may be able to augment the antidepressant effects of TMS. tsTMS was equally well-tolerated to TMS-alone.
Conclusions
Our next step is a pilot of multiple sessions of tsTMS with patients, to obtain further mechanistic evidence and estimates of clinical effect-size.
References
1Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:369-388. doi:10.1146/annurev-publhealth-031912-114409
2Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201-206. doi:10.1016/j.neuron.2004.12.033
3López-Alonso V, Cheeran B, Río-Rodríguez D, Fernández-Del-Olmo M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014;7(3):372-380. doi:10.1016/j.brs.2014.02.004
4Kaster TS, Downar J, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, Knyahnytska Y, Kennedy SH, Lam RW, Daskalakis ZJ, Blumberger DM. Trajectories of response to dorsolateral prefrontal rTMS in major depression: A three-D study. Am J Psychiatry. 2019;176(5):367-375. doi:10.1176/appi.ajp.2018.18091096
5To WT, de Ridder D, Hart J, Vanneste S. Changing brain networks through non-invasive neuromodulation. Front Hum Neurosci. 2018;12:128. doi:10.3389/fnhum.2018.00128
6Spronk D, Arns M, Barnett KJ, Cooper NJ, Gordon E. An investigation of EEG, genetic and cognitive markers of treatment response to antidepressant medication in patients with major depressive disorder: A pilot study. J Affect Disord. 2011;128(1-2):41-48. doi:10.1016/j.jad.2010.06.021
7Li CT, Hsieh JC, Huang HH, et al. Cognition-modulated frontal activity in prediction and augmentation of antidepressant efficacy: a randomized controlled pilot study. Cereb Cortex. 2016;26(1). doi:10.1093/cercor/bhu191
8Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200-206. doi:10.1192/bjp.178.3.200
9McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119:1-8. doi:10.1016/j.jad.2009.04.022
10Hosseinian T, Yavari F, Biagi MC, et al. External induction and stabilization of brain oscillations in the human. Brain Stimul. 2021;14(3):579-587. doi:10.1016/j.brs.2021.03.011
11Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59(9):816-820. doi:10.1016/j.biopsych.2005.10.015
12Harmer CJ, Cowen PJ. “It's the way that you look at it”—a cognitive neuropsychological account of SSRI action in depression. Philos Trans R Soc B: Biol Sci. 2013;368:201204073. doi:10.1098/rstb.2012.0407.
Learning Objectives
1. Gain awareness of current use of TMS in major depressive disorder
2. Understand how tACS-synchronisation can be achieved alongside TMS ("tsTMS")
3. Understand how tsTMS modifies markers of potential antidepressant efficacy (oscillatory activity, emotional bias)
O025 - EVOKED COMPOUND ACTION POTENTIAL-CONTROLLED CLOSED-LOOP SPINAL CORD STIMULATION PRODUCES ANALGESIA IN RATS WITH NEUROPATHIC PAIN (ID 210)
Abstract
Introduction
Evoked Compound Action Potentials (ECAPs) represent the neural recruitment of Aβ-fibers in the dorsal column following spinal cord stimulation (SCS)1. In humans, ECAP recordings have been incorporated into a closed-loop SCS (CL-SCS) system that automatically adjusts stimulation intensity to maintain a target ECAP amplitude. Importantly, ECAP-controlled CL-SCS can be superior to open-loop SCS systems in humans2,3. We recently showed that ECAPs can be reliably recorded from naive rats4. Here, we demonstrate for the first time the application of ECAP-controlled CL-SCS in neuropathic rats and its efficacy for pain relief.
Materials / Methods
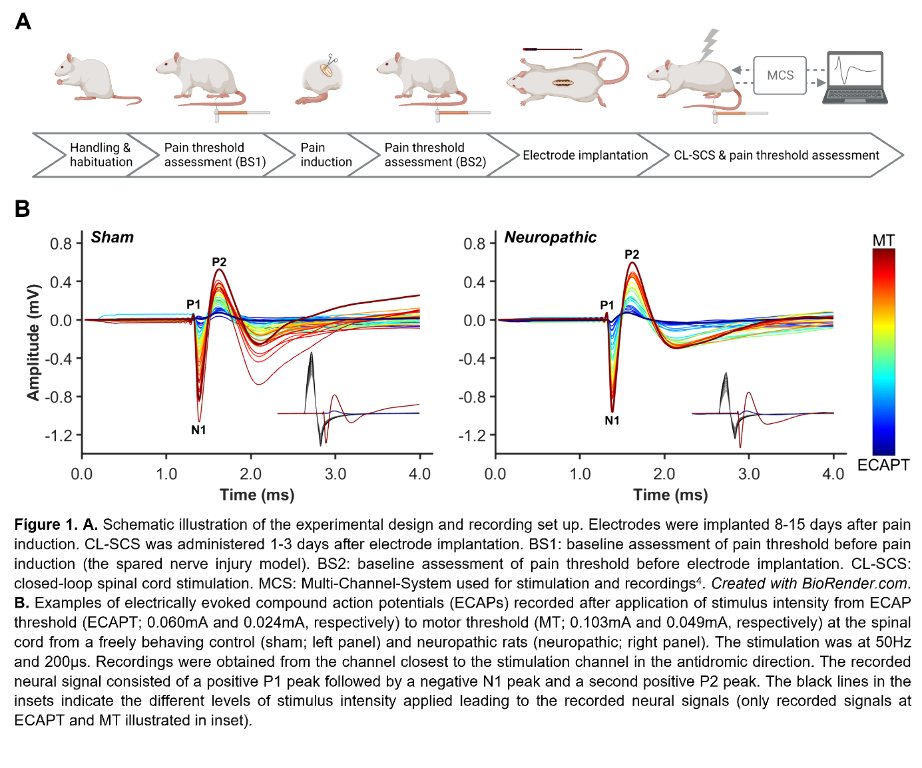
Figure 1A illustrates the experimental steps. Adult male Sprague–Dawley rats (200-300g) were subjected to neuropathic pain using the spared nerve injury model. A custom-made six-contact electrode4 was implanted epidurally covering T11-L3, as confirmed by CT or X-ray. Stimulation and recording were performed in freely behaving animals as previously described4. One contact was used for stimulation and recordings were made from the remaining contacts. The CL-SCS was delivered at 50Hz and 200µs for 30 minutes. To determine analgesic effects from stimulation, paw withdrawal thresholds to mechanical stimuli were assessed using the von Frey test. Protocols were approved by the UK Home Office.
Results
Figure 1B shows ECAPs recorded from neuropathic and sham (control) rats using 50Hz and 200µs stimulation. As expected, the recorded ECAPs showed a characteristic triphasic morphology and the amplitude (P2-N1, mV) increased as higher currents (mA) were applied. Figure 2A illustrates that these recordings allowed for successful application of ECAP-controlled CL-SCS in freely behaving neuropathic and sham rats. The analgesic effects of CL-SCS are shown in Figure 2B. It provided a significant reduction of mechanical hypersensitivity assessed by the von Frey test. The strongest analgesic effect was observed during the application of CL-SCS. However, after the termination of CL-SCS the effect gradually declined up to 30 minutes after the application of CL-SCS. CL-SCS had no effect in sham rats not subjected to neuropathic pain.
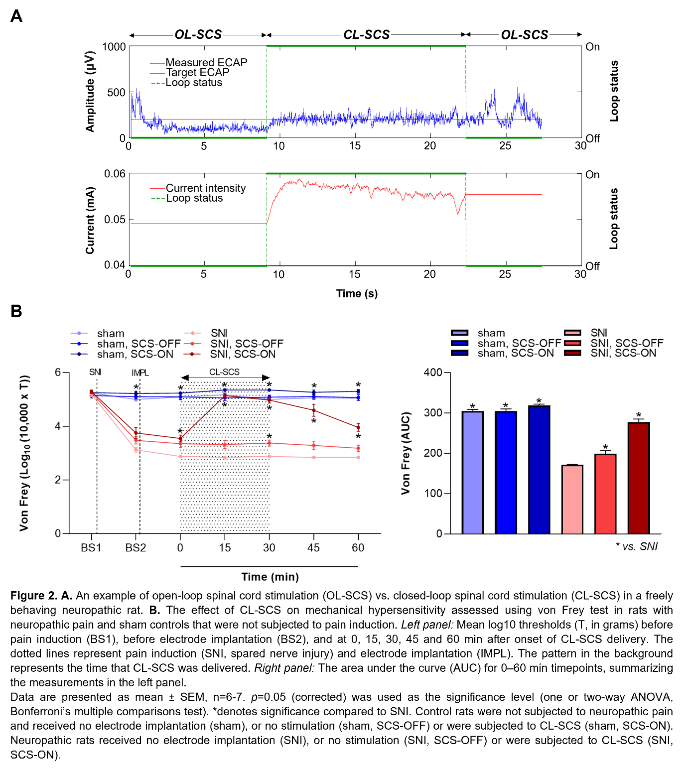
Discussion
This is the first demonstration of analgesic effects resulting from the application of ECAP-controlled CL-SCS in neuropathic rats.
Conclusions
This novel observation, together with our published work on the reliability of ECAP recordings in rats4, allow better translation of pre-clinical SCS models as our method is more closely aligned to human stimulation protocols. Our results also elucidate the mechanisms of SCS action to further improve future clinical SCS applications.
References
(1) Parker, J., Karantonis, D. & Single, P. Hypothesis for the mechanism of action of ECAP-controlled closed-loop systems for spinal cord stimulation. Healthc Technol Lett 7, 76–80 (2020).
(2) Mekhail, N. et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol 19, 123–134 (2020).
(3) Mekhail, N. et al. Durability of Clinical and Quality-of-Life Outcomes of Closed-Loop Spinal Cord Stimulation for Chronic Back and Leg Pain: A Secondary Analysis of the Evoke Randomized Clinical Trial. JAMA Neurol 79, 251–260 (2022).
(4) Dietz, B. E., Mugan, D., Vuong, Q. C. & Obara, I. Electrically Evoked Compound Action Potentials in Spinal Cord Stimulation: Implications for Preclinical Research Models. Neuromodulation 25, 64–74 (2022).
Learning Objectives
(1) Describe the novel approach allowing reliable recording of ECAPs from the dorsal column axons in freely behaving neuropathic and control rats.
(2) Interpret experimental outcomes showing that ECAP-controlled CL-SCS produces analgesia in neuropathic rats.
(3) Explain how the use of ECAP-controlled CL-SCS in animal models may enable better translations between pre-clinical and clinical research in SCS.
O026 - TRANSCRANIAL DIRECT CURRENT STIMULATION (TDCS) MODULATES CORTICAL NETWORKS DURING VISIOMOTOR LEARNING IN SCHIZOPHRENIA (ID 155)
Abstract
Introduction
Transcranial Direct Current Stimulation (tDCS) is a non-invasive brain stimulation approach in which low level currents are applied across the scalp to influence brain function (rev. in1). tDCS and other non-invasive brain stimulation approaches have the potential to increase plasticity and enhance cortical function2, enhance neurorehabilitation3, and reverse deficits in neuropsychiatric disorders such as schizophrenia4. While most studies have focused on its effects on local excitability (for discussion see5), more recent studies have focused on network-level effects across cortical regions6. Here, we used the high temporal resolution of EEG combined with a well-studied (rev. in7) visuo-motor learning paradigm – the serial reaction time task (SRTT) - to investigate cortical network mechanisms underlying tDCS effects in patients with schizophrenia.
Materials / Methods
Eighteen patients with schizophrenia and seventeen matched controls, aged 19–54, participated in the study. During the EEG, subjects performed the SRTT, consisting of presentation of a 5-element repeat sequence of color-coded visual cues, and were asked to press one of four color-coded keys as quickly as possible following presentation of each visual cue. During the EEG, subjects received four stimulation conditions on separate days (Sham, Motor- cathodal, Visual-cathodal, Motor-anodal) using a constant current of 2 mA intensity applied for 30 min during task performance.
Results
We analyzed mean RT as a function of condition across groups during stimulation and found a highly significant effect of Group, stimulation condition and Group X Condition interaction. In EEG, we used a source-space analysis approach to evaluate pairwise beta-frequency (12-18 Hz) coherence between dorsal-visual, SMA and motor nodes of the canonical SRTT circuit8,9 as a function both of motor learning and tDCS effect. In controls, motor-cathodal stimulation and visual-cathodal stimulation significantly modulated the coherence across motor cortex and SMA. In patients, motor-cathodal stimulation significantly modulated the coherence between SMA and visual cortex, visual-cathodal stimulation significantly modulated the coherence across motor cortex and SMA and between motor cortex and visual cortex.
Discussion
We observed robust effects when EEG responses were mapped into source space. In particular, beta-coherence measures were sensitive to the differential network modulatory effects of tDCS when comparing healthy controls and patients. In addition to its specific findings regarding tDCS modulation of motor learning, the present study may also have direct implications for use of network-targeted tDCS for treatment of motor manifestations of neuropsychiatric disorders in general.
Conclusions
We demonstrate that source-level beta-coherence measures obtained concurrent with tDCS may serve as effective target engagement biomarker for motor learning.
References
1- Brunoni, A. R. et al. Understanding tDCS effects in schizophrenia: a systematic review of clinical data and an integrated computation modeling analysis. Expert review of medical devices 11, 383-394, doi:10.1586/17434440.2014.911082 (2014).
2- Nissim, N. R. et al. Effects of Transcranial Direct Current Stimulation Paired With Cognitive Training on Functional Connectivity of the Working Memory Network in Older Adults. Frontiers in Aging Neuroscience 11, doi:10.3389/fnagi.2019.00340 (2019).
3- Sánchez-Kuhn, A., Pérez-Fernández, C., Cánovas, R., Flores, P. & Sánchez-Santed, F. Transcranial direct current stimulation as a motor neurorehabilitation tool: an empirical review. BioMedical Engineering OnLine 16, doi:10.1186/s12938-017-0361-8 (2017).
4- Brunelin, J. et al. Examining Transcranial Direct-Current Stimulation (tDCS) as a Treatment for Hallucinations in Schizophrenia. American Journal of Psychiatry 169, 719-724, doi:10.1176/appi.ajp.2012.11071091 (2012).
5- Polanía, R., Nitsche, M. A. & Ruff, C. C. Studying and modifying brain function with non-invasive brain stimulation. Nature Neuroscience 21, 174-187, doi:10.1038/s41593-017-0054-4 (2018).
6- Sehatpour, P. et al. Network-level mechanisms underlying effects of transcranial direct current stimulation (tDCS) on visuomotor learning. NeuroImage, 117311, doi:10.1016/j.neuroimage.2020.117311 (2020).
7- Buch, E. R. et al. Effects of tDCS on motor learning and memory formation: A consensus and critical position paper. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 128, 589-603, doi:10.1016/j.clinph.2017.01.004 (2017).
8- Keele, S. W., Ivry, R., Mayr, U., Hazeltine, E. & Heuer, H. The cognitive and neural architecture of sequence representation. 110, 316-339, doi:10.1037/0033-295x.110.2.316 (2003).
9- Kantak, S. S., Mummidisetty, C. K. & Stinear, J. W. Primary motor and premotor cortex in implicit sequence learning--evidence for competition between implicit and explicit human motor memory systems. The European journal of neuroscience 36, 2710-2715, doi:10.1111/j.1460-9568.2012.08175.x (2012).
Learning Objectives
1- To characterize the underlying network-level mechanisms of action of tDCS in improving motor learning in schizophrenia and related neuropsychiatric disorders.
2- To characterize the differential network effects of tDCS between healthy controls and patients with schizophrenia.
3- To investigate the cortical network effects of tDCS as potential target engagement biomarkers during clinical treatment studies.
