O024 - DEVELOPING AN AUGMENTATION APPROACH FOR ITBS IN MAJOR DEPRESSIVE DISORDER USING SYNCHRONISED TACS (ID 106)
- Paul M. Briley (United Kingdom)
Abstract
Introduction
Transcranial magnetic stimulation (TMS) is approved for treating major-depressive-disorder (MDD), a condition that affects 1 in 7 people during their lifetime1. An increasingly popular protocol, due to short session time, is intermittent theta burst stimulation (iTBS), in which TMS pulse triplets repeat at 5Hz (a “theta” frequency)2. Response to TMS, including iTBS, is highly variable3,4. We are examining a relatively inexpensive, easy-to-implement, approach to make iTBS act faster, for more people, by modifying “brain state” at the time of stimulation.
Materials / Methods
One aspect of “brain state” is oscillatory activity, generated by neural-circuit dynamics. Oscillatory activity mediates excitability within brain areas and connectivity between areas5. Pre-treatment fronto-central theta oscillatory power, measured with electroencephalography (EEG), is correlated with response to antidepressant medications6. Li et al.7 found that performance of a theta-eliciting task prior to TMS was associated with better clinical response, and post-task theta predicted response. However, task-based augmentation is unsuitable for people with more severe depression, characterised by cognitive and motivational difficulties8,9.
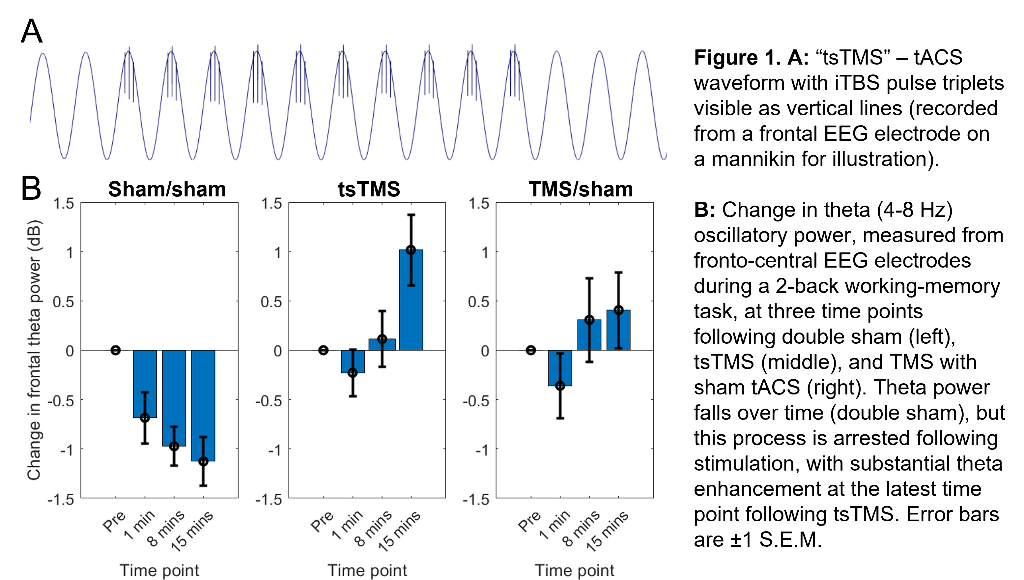
Our approach seeks to enhance theta activity during and after iTBS (and thus augment iTBS efficacy) using concurrent, synchronised, transcranial alternating-current stimulation (tACS). (Building on work showing combined tACS-TMS enhances oscillatory power for traditional “rTMS” protocols10). tACS is delivered at a theta-frequency (5Hz), with 1mA peak-to-peak amplitude, using 9cm2 round-electrodes at F3 (anode) and TP9 (cathode). iTBS at 80% resting-motor-threshold (20 cycles - 600 pulses, 3.3 minutes) is delivered via a 70mm figure-eight coil over F3, with pulse triplets timed to occur at the tACS peaks (Fig.1A).
Results
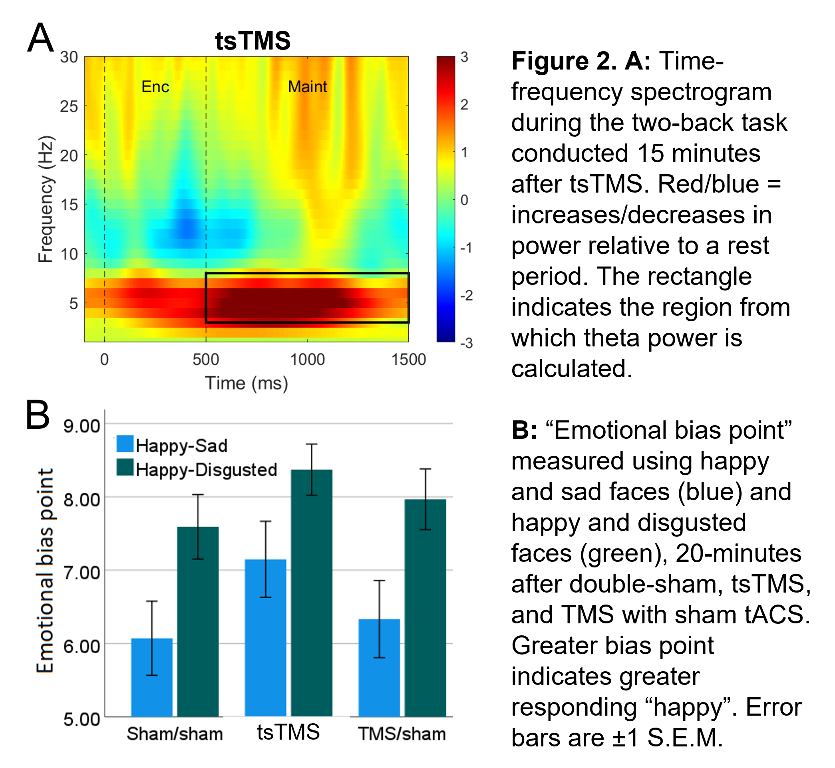
In an ongoing non-patient study (current N=12, 6 female, age±SD 24.1±5.6 years), theta activity during an n-back working-memory task is recorded before, and 1/8/15-minutes after, one session of “tACS-synchronised TMS” (tsTMS), TMS with sham-tACS, or double-sham. In the double-sham condition, theta decreased with repetition of the n-back task. In both stimulation conditions, this reduction was arrested, with an increase in theta (relative to pre-stimulation) at the 8/15-minute time points. At 15-minutes, theta was greatest in the tsTMS condition (Fig.1B,2A). At 20-minutes, positive emotional bias (a marker of potential antidepressant efficacy11,12, obtained from ratings of “happy” or “sad” to briefly-presented facial expressions) was greatest after tsTMS (followed by TMS alone, Fig.2B).
Discussion
The results suggest tACS-synchronisation may be able to augment the antidepressant effects of TMS. tsTMS was equally well-tolerated to TMS-alone.
Conclusions
Our next step is a pilot of multiple sessions of tsTMS with patients, to obtain further mechanistic evidence and estimates of clinical effect-size.
References
1Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:369-388. doi:10.1146/annurev-publhealth-031912-114409
2Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201-206. doi:10.1016/j.neuron.2004.12.033
3López-Alonso V, Cheeran B, Río-Rodríguez D, Fernández-Del-Olmo M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014;7(3):372-380. doi:10.1016/j.brs.2014.02.004
4Kaster TS, Downar J, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, Knyahnytska Y, Kennedy SH, Lam RW, Daskalakis ZJ, Blumberger DM. Trajectories of response to dorsolateral prefrontal rTMS in major depression: A three-D study. Am J Psychiatry. 2019;176(5):367-375. doi:10.1176/appi.ajp.2018.18091096
5To WT, de Ridder D, Hart J, Vanneste S. Changing brain networks through non-invasive neuromodulation. Front Hum Neurosci. 2018;12:128. doi:10.3389/fnhum.2018.00128
6Spronk D, Arns M, Barnett KJ, Cooper NJ, Gordon E. An investigation of EEG, genetic and cognitive markers of treatment response to antidepressant medication in patients with major depressive disorder: A pilot study. J Affect Disord. 2011;128(1-2):41-48. doi:10.1016/j.jad.2010.06.021
7Li CT, Hsieh JC, Huang HH, et al. Cognition-modulated frontal activity in prediction and augmentation of antidepressant efficacy: a randomized controlled pilot study. Cereb Cortex. 2016;26(1). doi:10.1093/cercor/bhu191
8Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200-206. doi:10.1192/bjp.178.3.200
9McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119:1-8. doi:10.1016/j.jad.2009.04.022
10Hosseinian T, Yavari F, Biagi MC, et al. External induction and stabilization of brain oscillations in the human. Brain Stimul. 2021;14(3):579-587. doi:10.1016/j.brs.2021.03.011
11Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59(9):816-820. doi:10.1016/j.biopsych.2005.10.015
12Harmer CJ, Cowen PJ. “It's the way that you look at it”—a cognitive neuropsychological account of SSRI action in depression. Philos Trans R Soc B: Biol Sci. 2013;368:201204073. doi:10.1098/rstb.2012.0407.
Learning Objectives
1. Gain awareness of current use of TMS in major depressive disorder
2. Understand how tACS-synchronisation can be achieved alongside TMS ("tsTMS")
3. Understand how tsTMS modifies markers of potential antidepressant efficacy (oscillatory activity, emotional bias)