Welcome to the e-INS 2023 Interactive Program
123 Presentations
REAL-WORLD OUTCOMES USING DBS SYSTEMS WITH DIRECTIONALITY AND MULTIPLE INDEPENDENT CURRENT CONTROL: EXPERIENCE IN THE USA (ID 34)
Abstract
Introduction
Deep Brain Stimulation (DBS) has been substantiated by several randomized controlled trials as an effective strategy for reducing the motor complications in Parkinson's disease (PD).1-3 This motor improvement has been shown in cohorts to possibly be sustained for up to 10 years.4 Clinical data collected from a wide variety of implanting centers using local standard of care has revealed overall improvements in PD disease symptoms and quality-of-life when applying DBS therapy. Here, we present preliminary outcomes from an ongoing, prospective, multicenter study conducted in the United States of patients implanted with directional DBS Systems capable of multiple independent current control (MICC) for use in the management of the motor signs and symptoms of levodopa- responsive PD.
Materials / Methods
Prospectively-enrolled participants were implanted with a DBS system (VerciseTM, Boston Scientific, Valencia, CA, USA), a multiple-source, constant- current device, and were assessed up to 3-years post-implantation. Clinical measures recorded at baseline and during study follow-up included: MDS-Unified Parkinson's disease Rating Scale (MDS-UPDRS), Parkinson's Disease Questionnaire (PDQ-39), Global Impression of Change (GIC), and Non-Motor Symptom Assessment Scale (NMSS), and adverse events.
Results
A total of 111-patients (mean age: 64.1 ± 8.7 years, 73% male, disease duration 9.7 ± 5.3 years, n = 108) have been enrolled to date, and 93 have devices which have been activated. A 56.4% improvement (28.2-points, p<0.0001) in motor function was noted at 6-months as assessed by MDS-UPDRS III in the meds "off" stimulation “on” condition. Quality of life was improved with an 8.4-point change in the PDQ-39 Summary Index (p<0.0001). This change exceeded the minimal clinically important difference (MCID) for PDQ-39 which is 4.7-points.5 At 6-months post-DBS, a categorical subject measure revealed that 98% of patients and 95% of clinicians reported improvements (GIC). There have been no lead breakages.
Discussion
In this study, collection of real-world data across multiple implanting centers is informing on longer-term outcomes of MICC-based DBS systems.
Conclusions
Real-world outcomes from this large, prospective, multicenter outcomes study demonstrate improvement in quality-of-life and motor function following DBS, and overall satisfaction among patients and clinicians. Data from this study will continue to provide insight regarding the application of the MICC-based directional DBS Systems for PD in clinical practice.
References
1. Okun MS, Gallo BV, Mandybur G, et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson's disease: an open-label randomised controlled trial. Lancet Neurol. 2012 Feb;11(2):140-9.
2. Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med. 2013 Feb 14;368(7):610-22.
3. Vitek JL, Jain R, Chen L, Tröster AI, et al. Subthalamic nucleus deep brain stimulation with a multiple independent constant current-controlled device in Parkinson's disease (INTREPID): a multicentre, double-blind, randomised, sham-controlled study. Lancet Neurol. 2020 Jun;19(6):491-501.
4. Deuschl G, Paschen S, Witt K. Clinical outcome of deep brain stimulation for Parkinson's disease. Handb Clin Neurol. 2013;116:107-28.
5. Horváth K, Aschermann Z, Kovács M, et al. Changes in Quality of Life in Parkinson's Disease: How Large Must They Be to Be Relevant? Neuroepidemiology. 2017;48(1-2):1-8.
Learning Objectives
1. To assess real-world motor function outcomes when utilizing MICC-based DBS systems with directionality in patients with Parkinson’s Disease.
2. To assess real-world safety when utilizing MICC-based DBS systems with directionality on patients with Parkinson’s Disease.
3. To assess real-world quality-of-life outcomes when utilizing MICC-based DBS systems with directionality on patients with Parkinson’s Disease.
O001 - ADVANCED APPLICATIONS OF EVOKED COMPOUND ACTION POTENTIAL SENSING: QUANTIFYING MECHANISTIC AND DOSING DIFFERENCES BETWEEN BURST AND CONVENTIONAL SPINAL CORD STIMULATION IN OVINES (ID 40)
Abstract
Introduction
Unlike conventional spinal cord stimulation (SCS)—which employs single pulses delivered at a fixed rate to the dorsal spinal cord—burst SCS uses a fixed rate, five-pulse burst of stimuli as a treatment for chronic pain. The electrical charge per second (i.e., the battery depletion) is three times greater with burst SCS although burst SCS is generally programmed at lower amplitudes compared to conventional SCS.1 Mechanistic explanations have suggested burst SCS differentially modulates the medial and lateral pain pathways versus conventional SCS.2 Differences in neural activation resulting from either burst or conventional SCS may be quantified with the spinal evoked compound action potential (ECAP), an electrical measure of synchronous neural activation. Here, we use ECAPs acquired from both the ALS and dorsal columns in sheep to assess these differences and gain mechanistic insight into both types of SCS.
Materials / Methods
Seven sheep were each implanted with a dorsal stimulation lead at T9/T10, a dorsal ECAP sensing lead at T6/T7, and a lead also at T9/T10 but adjacent to the ALS (Fig. 1). Both burst and conventional SCS with swept stimulation amplitudes up to the visual motor threshold (vMT) were delivered to three different dorsal spinal locations,3 and ECAP thresholds (ECAPTs) were calculated for all combinations.4 Then, changes in ALS activation for both types of SCS was assessed using test stimulation delivered to the ALS.

Fig. 1
Results
The ALS ECAP recordings were separated into three different bins per stimulation location for both the burst and conventional dorsal SCS—sub-ECAPT, sub-ECAPT to vMT, and supra-vMT (Fig. 2). In all cases, no significant difference (p > 0.05) was noted between burst and conventional SCS for these three bins for all three stimulation sites. Further, both burst and conventional SCS potentiated ALS ECAPs in an equivalent manner as stimulation amplitudes were increased.

Fig. 2
Discussion
When dosed equivalently relative to the ECAPT—a measure that correlates with the perception threshold—burst SCS does not result in differentially unique changes in ALS activation versus conventional SCS; additionally, burst SCS below the ECAPT does not result in any discernable excitability changes in the neural pathways feeding pain relevant supraspinal sites.
Conclusions
Differences noted previously between burst and conventional SCS results (i.e., in terms of clinical benefit)5 may simply result from non-equivalent dosing between these stimulation modalities.
References
1De Ridder D, Vanneste S, Plazier M, Van Der Loo E, Menovsky T. Burst spinal cord stimulation: Toward paresthesia-free pain suppression. Neurosurgery. 2010;66(5):986-990. doi:10.1227/01.NEU.0000368153.44883.B3
2De Ridder D, Vanneste S. Burst and Tonic Spinal Cord Stimulation: Different and Common Brain Mechanisms. Neuromodulation. 2016;19(1):47-59. doi:10.1111/ner.12368
3Al-Kaisy A, Baranidharan G, Palmisani S, et al. Comparison of Paresthesia Mapping to Anatomical Placement in Burst Spinal Cord Stimulation: Initial Trial Results of the Prospective, Multicenter, Randomized, Double-Blinded, Crossover, CRISP Study. Neuromodulation. 2020;23(5):613-619. doi:10.1111/ner.13104
4Pilitsis JG, Chakravarthy K V, Will AJ, et al. The Evoked Compound Action Potential as a Predictor for Perception in Chronic Pain Patients: Tools for Automatic Spinal Cord Stimulator Programming and Control. Front Neurosci. 2021;15:881. doi:10.3389/fnins.2021.673998
5Deer T, Slavin K V, Amirdelfan K, et al. Success Using Neuromodulation With BURST (SUNBURST) Study: Results From a Prospective, Randomized Controlled Trial Using a Novel Burst Waveform. Neuromodulation. 2018;21(1):56-66. doi:10.1111/ner.12698
Learning Objectives
1. Differences between burst and conventional SCS; burst SCS consists of a cluster of 5 - 1 ms wide pulses delivered at 40 Hz, while conventional stimulation is a single pulse (but may also be of the same pulsewidth and frequency). Previous mechanistic discussions have described a differentially unique effect of burst SCS on the lateral and medial pain pathway.
2. ECAPs may be used as a quantitative measure of neural activation, not just from the dorsal columns but from the anterolateral tracts as well.
3. When dosed equivalently using ECAPs, no differentially unique effect is noted with burst versus conventional SCS. Mechanistic differences noted previously between burst and conventional SCS may have resulted from non-equivalent dosing between the stimulation paradigms.
O006 - COMPARING SCS AND CONVENTIONAL MEDICAL MANAGEMENT IN PATIENTS WITH NO PRIOR BACK SURGERY (SOLIS RCT) (ID 50)
Abstract
Introduction
Spinal Cord Stimulation (SCS) as a treatment for chronic pain has been historically designated for patients who have had at least one prior spinal surgery. Considering the opioid crisis, and the often-mixed clinical success of conservative treatment approaches and invasive back surgery procedures, there is growing interest in utilizing SCS in chronic pain patients who have not yet undergone previous surgical intervention.1-4 Recent SCS devices offer substantially more novel technological or neurostimulative capabilities than older-generational SCS systems. Correspondingly, interventional treatment approaches capable of multimodal therapeutic strategies are now actively recommended by pain care advocates.5,6
Materials / Methods
This is a prospective, multicenter randomized, controlled study (SOLIS) that compares SCS (WavewriterTM SCS Systems, Boston Scientific, Valencia, CA, USA) versus Conventional Medical Management (CMM) in patients with chronic low back and/or leg pain with no prior spinal surgery (Clinicaltrials.gov: NCT04676022). Enrolled non-surgical back pain (NSBP) patients who met inclusion criteria were randomized to SCS versus CMM. Key inclusion criteria include diagnosis of chronic low back pain, with or without leg pain, for ≥6 months, and documented care of chronic pain for ≥90 days. The primary endpoint is responder rate (≥ 50% reduction in pain) with no increase in baseline opioid medications to treat pain at 3-months following treatment activation. Other secondary and/or exploratory measures include Quality-of-Life (SF-36; EQ-5D-5L), Disability (Oswestry Disability Index, ODI), and Safety Outcomes.
Results
The study successfully met its primary endpoint (p < 0.0001) and secondary endpoints based on a prespecified cohort of 60 treatment activated subjects. The primary endpoint analysis demonstrated that multimodal SCS combined with CMM was superior to CMM alone (p<0.0001) in treating NSBP patients at 3-months follow-up (SCS: 88% versus CMM: 8%). A 27-point reduction in ODI score (improvement in disability) was noted in the SCS group in comparison to a 6-point reduction in the CMM group. Ninety-two percent of subjects with SCS reported treatment satisfaction (i.e., much, or very much improved) at 3-months versus only 6% in the CMM group.
Discussion
Given the prevalence of non-surgical, refractory back pain and the increasing economic and societal burden it poses, providing SCS as an additional tool within the therapeutic armamentarium for chronic pain represents a key opportunity to address a clinically important need.
Conclusions
SCS with multiple modalities is effective in treating chronic pain in patients with no prior back surgery demonstrating superior outcomes compared with CMM. SOLIS RCT outcomes are consistent with those reported in a preceding RCT assessing patients diagnosed with currently-approved “on-label” chronic pain indications.7
References
1. Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015 Feb;14(2):162-73.
2. Al-Kaisy A, Van Buyten JP, Kapural L, Amirdelfan K, Gliner B, Caraway D, Subbaroyan J, Edgar D, Rotte A. 10 kHz spinal cord stimulation for the treatment of non-surgical refractory back pain: subanalysis of pooled data from two prospective studies. Anaesthesia. 2020 Jun;75(6):775-784.
3. Kapural L, Jameson J, Johnson C, Kloster D, Calodney A, Kosek P, Pilitsis J, Bendel M, Petersen E, Wu C, Cherry T, Lad SP, Yu C, Sayed D, Goree J, Lyons MK, Sack A, Bruce D, Rubenstein F, Province-Azalde R, Caraway D, Patel NP. Treatment of nonsurgical refractory back pain with high-frequency spinal cord stimulation at 10 kHz: 12-month results of a pragmatic, multicenter, randomized controlled trial. J Neurosurg Spine. 2022 Feb 11:1-12.
4. Kapural L, Calodney A. Retrospective Efficacy and Cost-Containment Assessment of 10 kHz Spinal Cord Stimulation (SCS) in Non-Surgical Refractory Back Pain Patients. J Pain Res. 2022 Nov 16;15:3589-3595.
5. U.S. Department of Health and Human Services, Alliance to Advance Comprehensive Integrative Pain Management (2019). Pain Management Best Practices Inter-Agency Task Force Report. https://painmanagementalliance.org/resources/hhs-report-2019/. Accessed October 28th, 2022.
6. Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical Practice Guideline for Prescribing Opioids for Pain - United States, 2022. MMWR Recomm Rep. 2022 Nov 4;71(3):1-95.
7. Wallace MS., et.al. Combination Therapy with Simultaneous Delivery of Spinal Cord Stimulation Modalities: COMBO Randomized Controlled Trial [Abstract]. NANS Annual Meeting, 2022.
Learning Objectives
1. To evaluate the use of SCS in the treatment of patients with non-surgical back pain (NSBP)
2. To compare pain relief outcomes of SCS versus Conventional Medical Management in a prospective randomized controlled trial for NSBP patients.
3, To compare quality-of-life outcomes of SCS versus Conventional Medical Management in a prospective randomized controlled trial for NSBP patients.
O008 - CLINICAL OUTCOMES OF A NOVEL, FAST-ACTING SUB-PERCEPTION SCS THERAPY ENGAGING SURROUND INHIBITION (FAST PROSPECTIVE STUDY) (ID 54)
Abstract
Introduction
Fast-Acting Sub-Perception Therapy (FAST) has demonstrated robust, profound pain relief with rapid (seconds to minutes) onset of analgesia in chronic pain patients implanted with Spinal Cord Stimulation (SCS) systems.1 Initial results have been corroborated at other centers.2 Sustained long-term improvement of up to 3 years has also been reported in an observational, real-world case-series.3 Recently published work suggests that FAST engages the surround inhibition mechanism of action, and computational modeling suggests that FAST activates dorsal column axons and inhibits dorsal horn projection neurons.4
Materials / Methods
The FAST study is a prospective, multi-center, single-arm study (with adaptive design) of patients implanted with SCS systems (WaveWriterTM Systems, Boston Scientific, Valencia, CA, USA) for chronic pain. The primary endpoint is based on the targeted pain responder rate (≥50% reduction) 3-months post-activation with no increase in average daily opioid medications. Secondary endpoints include (but are not limited to) patient satisfaction (Patient Global Impression of Change, PGIC) and other functional outcomes including disability (Oswestry Disability Index, ODI) and sleep. Key inclusion criteria include diagnosis of predominantly neuropathic pain of trunk and/or limbs for at least 6- months, and no back surgery within 6-months prior to screening.
Results
The study successfully met its primary endpoint (p<0.0001) based on a prespecified cohort of 20 subjects. A 6.1-point reduction (p<0.0001) in mean low back pain score at 3 months was reported with a 95% responder rate (≥50% improvement in pain relief). A 31-point improvement in disability (ODI) and high patient satisfaction ratings (85% reported much improved or very much improved, PGIC) were found. At the FAST-SCS activation visit, FAST responders achieved maximum paresthesia-free pain relief within a mean of 5.4-minutes.
Discussion
Preliminary results from this ongoing FAST prospective study suggests that profound, significant pain relief along with improvement in functional outcomes may be achieved in chronic pain patients with FAST therapy and additional SCS therapy options. With availability of multiple modalities in SCS systems, the capability for rapid onset of analgesia is particularly useful in evaluating what may be best suited for patients.
Conclusions
Significant pain relief and improvement in functional outcomes were reported in the FAST prospective study. These study results are consistent with published real-world experience that has assessed over 200 patients.5
References
1. Metzger CS, et al. A novel fast-acting sub-perception spinal cord stimulation therapy enables rapid onset of analgesia in patients with chronic pain. Expert Rev Med Devices 2021 Mar; 18(3): 299-306.
2. Ferro R, Pei Y, Jain R. Significant Pain Relief Using an SCS System Delivering Novel, Fast-Acting Sub-Perception Therapy [Abstract e-poster] Annual Meeting of the North American Neuromodulation Society, 2023.
3. Bayerl S, Paz Solis JF, Matis G, et al. Clinical Outcomes Using A New Fast Acting Sub Perception Therapy For Chronic Pain A Multicenter European Observational Real World Study [Abstract Poster 113]. Annual Meeting of the North American Neuromodulation Society, 2023.
4. Gilbert JE, Titus N, Zhang T, Esteller R, Grill WM. Surround Inhibition Mediates Pain Relief by Low Amplitude Spinal Cord Stimulation Modeling and Measurement. eNeuro 2022. Sep 22.ENEURO.0058-22.
5. Metzger C, Hammond MB, Ferro R, et al. Outcomes of a Large, Multicenter, Real-World Study using an SCS System Engaging Surround Inhibition [Abstract Poster 80]. Annual Meeting of the North American Neuromodulation Society, 2023.
Learning Objectives
1. To assess the FAST-SCS responder rate (defined as >50% pain relief when using FAST-SCS) among patients implanted with an SCS system
2. To assess the duration of onset of pain relief following FAST-SCS activation in FAST-SCS responders
3. To assess pain relief and functional outcomes in patients with an SCS system capable of FAST-SCS.
O009 - IMPROVED PAIN OUTCOMES AND THERAPY LONGEVITY AFTER SALVAGE USING NOVEL SCS SYSTEMS: EUROPEAN EXPERIENCE (ID 64)
Abstract
Introduction
Spinal Cord Stimulation (SCS) is an effective therapy for chronic neuropathic pain, with its long-term efficacy well-established. However, some patients experience loss of efficacy (LoE) over time and become refractory over the course of follow-up.1 Providing various waveforms and programming options in novel SCS systems can facilitate more customized delivery of analgesic neurostimulation to chronic pain patients implanted with an SCS device. However, technologies that offer such optimization capabilities are not accessible to long-term implanted patients using older devices, some of whom may experience loss or attenuation in therapeutic efficacy over time. These patients therefore may elect to undergo a "conversion" to a different SCS system that possesses these capabilities.
Materials / Methods
This is a real-world, multicenter retrospective study of patients who were previously implanted with an SCS system (commercially-available device) who went on to convert to a new device (Boston Scientific SCS Systems, Boston Scientific, Valencia, CA, USA) capable of multiple modality stimulation and/or combination therapy via an applicable device adaptor and new implantable pulse generator (IPG). Pain relief and other associated outcomes using both the previously-implanted SCS system and the newly connected device IPG are being collected.
Results
Fifty-one patients (mean age = 57.1 years) have been assessed to date. A mean baseline overall pain (NRS) score of 7.7 ± 2.0 (n = 40) was reported prior to receiving SCS. Sixty nine percent of patients (N = 35) chose to convert for better pain relief followed by the need to access multiple programs (35%) and/or to get coverage of new areas of pain (31%). Previous SCS devices represent a range of different manufacturers. Among all patients (regardless of reasons for conversion), a pain score of 3.4 was noted at last follow up and sustained improvement was noted up to 3.2 years with current systems. In patients for whom the conversion was performed to “rescue” suboptimal outcomes with the previous system (N=47), a mean 3.5-point improvement with the current system was noted at last follow-up (3.1 years post-implant, 7.0 ⇒ 3.6, p<0.0001).
Discussion
When experiencing problems with SCS device longevity and/or loss of efficacy, some previously-implanted patients may be able to obtain better outcomes using more advanced neuromodulation systems that offer a range of waveforms and programming options to address their chronic pain.
Conclusions
Significant improvement was noted in overall pain in patients who converted to a new SCS device capable of providing multiple device programming options including anatomically guided paresthesia-based stimulation, combination therapy, and novel sub-perception modalities.
References
1. Deer TR, Mekhail N, Provenzano D, et al Neuromodulation Appropriateness Consensus Committee The appropriate use of neurostimulation avoidance and treatment of complications of neurostimulation therapies for the treatment of chronic pain Neuromodulation Appropriateness Consensus Committee Neuromodulation 2014 Aug 17 6 571-97. discussion 597-8.
2. Kumar K, Hunter G, Demeria D Spinal cord stimulation in treatment of chronic benign pain challenges in treatment planning and present status, a 22-year experience Neurosurgery 2006 Mar 58 3 481 96 discussion 481-96.
Learning Objectives
1. To assess pain relief outcomes (including long-term outcomes) in an SCS-implanted population (with sub-optimal pain relief) who have replaced their older generation SCS batteries with a newer generation device that provides multiple modalities.
2. To assess the reasons for conversion in patients who converted to newer SCS systems.
3. To evaluate the programs that are most preferred by patients who have converted to a newer SCS system.
O021 - BILATERAL T12 DORSAL ROOT GANGLION STIMULATION FOR THE TREATMENT OF LOW BACK PAIN WITH 20HZ AND 4 HZ STIMULATION (ID 91)
Abstract
Introduction
Chronic Lumbar back pain (CLBP) is one of the most common chronic pain conditions resulting in both individual suffering and a burden to societies. For these patients there are several interventional treatment options such as surgery, blocks, radiofrequency, and spinal cord stimulation. Lately also Dorsal Root Ganglion Stimulation (DRG-S) has been mentioned as an option, by targeting bilateral T12 dorsal ganglia. In this study we will present the outcome of 11 patients with CLBP treated with bilateral T12 DRG-S.
Materials / Methods
13 patients with CLBP with and without leg pain were treated with bilateral T12 DRG-S. Three of the patients also received a third lumbar lead due to leg pain. 11 of the patients had a more than 50% pain relief during the per- or/and postoperative testing and received a fully implantable neurostimulator. Pain intensity, general health status, quality of life, pain catastrophizing, mental status, sleeping disorder, physical activity and patient satisfaction were followed using NRS, PROMIS-29, PCS, GAD-7, PHQ-9, ISI and patient satisfaction questionnaire at baseline before implantation, 3 months and 6 months. The results were analyzed based on 6 domains: pain relief, sleeping disorder, social ability, mental status, physical activity, and satisfaction. To be identified as a responder the patients should show a significant improvement in the pain relief domain together with at least two other domains. All responders were given the opportunity to test 4 Hz DRG-S and compare it with traditional 20 Hz stimulation.
Results
All 11 patients were identified as responders at six months. 5 of the patients had a more than 80% pain relief with an average NRS score reduction of 71 % for the whole group. Significant improvement could be observed in 3 domains for one patient, 4 domains for three patients, 5 domains for six patients and 6 domains for one patient. Seven patients chose to try 4 Hz stimulation. All seven identified 4 Hz stimulation at least as good or better than 20 Hz stimulation and chose to continue with 4 Hz stimulation.
Discussion
Bilateral T12 DRG-S seems to be an effective treatment for chronic low back pain with significant beneficial effect not only on pain but also on quality of life, pain catastrophizing, mental status, sleeping disorder and physical activity. 4 Hz DRG-S gave comparable or better result than 20 Hz stimulation.
Conclusions
T12 level could be a good target for DRG-S for CLBP. 4Hz DRG-S seems to be at least as good as 20Hz.
References
None
Learning Objectives
1. DRG-S could be a valuable treatment option for patients suffering from CLBP.
2. DRG-S have beneficial effects not only on pain but also other health metrics.
3. 4 Hz DRG-S seems to have comparable or at least as godd effect as 20 Hz stimulation.
O028 - LEGAL, ETHICAL, AND DEONTOLOGICAL CHALLENGES FOR CLINICAL INVESTIGATIONS IN NEUROMODULATION FOR CHRONIC PAIN (ID 95)
Abstract
Introduction
During the last century, a considerable number of ethics codes have emerged, sometimes embedding legally binding instruments, sometimes encoding good clinical practice for the first time. Apart from the general ethical considerations in use of neuromodulatory devices, specific ethical standards should be applied in clinical trials using neuromodulation as treatment option for chronic pain. The specific, but sometimes debatable place of neuromodulation in the treatment algorithm in chronic, refractory pain, makes the inclusion of such vulnerable populations in trials unique, but requires a solid framework to concur the ethical concerns.
Adding to the ethics guidelines, regulators have tried to keep up in protecting the patient, user and society of possible risks related to such devices, namely in the protection of the patient's privacy and ensuring manufacturer responsibility during the whole lifecycle of the device. This area of research with medical devices has become a regulatory minefield that the suggestion has been made to create an academic discipline of ‘medical device science’.
This review aims to demonstrate the limits of ethics guidelines, the personal responsibility of the researcher and the usefulness of the legal, ethics and deontology frameworks for clinical investigations in neuromodulation.
Materials / Methods
In this narrative review, the authors take the reader thought the full research cycle for clinical investigations in neuromodulation; from the initiation of the research towards execution of the research and publication. In each topic the authors provide practical advices building on a multidisciplinary experience in clinical neuromodulation practice, social researchers and legal and ethics advisors.
Results
The article focusses on common hurdles, namely patient centered study designs, obtaining true informed consent, clear contracts between sponsors and research partners, fees to the patient and post-trial accessibility and support of the device, usefulness of standards, including placebo and sham interventions, expectations with respect to institutional review boards or ethics committees, and publication hygiene.
Discussion
This review clearly demonstrated that general ethical guidelines and legislation need an additional translation towards specific fields to be useful for clinicians and researchers with a background in medical device science.
Conclusions
Due to the increased number of guidelines with respect to medical devices, awarness of these regulations should be increased, whereby legal experts could provide valuable input in the future developments of clinical trials in the field of neuromodulation.
References
None.
Learning Objectives
1. To create awarness about the set of regulations (legal and ethical) with respect to medical devices.
2. To learn about all aspects of medical ethics, and to know how to safeguard these principles in every step in clinical research from initiating an idea, over execution a study to reporting results.
3. To be able to point out the gaps in current legislation.
O010 - LONG-TERM EFFECTIVENESS OF SCS FOR ISCHEMIC PAIN DUE TO SEVERE PERIPHERAL VASCULAR DISEASE (RUTHERFORD 3, 4, AND 5) (ID 97)
Abstract
Introduction
Spinal cord stimulation (SCS) can improve pain relief, limb salvage, and microcirculatory blood flow in patients suffering from intractable ischemic pain due to peripheral vascular disease (PVD) who are not suitable for revascularization1. Herein, we present long-term effectiveness and safety outcomes using SCS for PVD at a single center.
Materials / Methods
51 patients (37 men [mean age 68.9 ± 10.2 y], 14 women [mean age (68.7 ± 14.6 y]) underwent SCS (n = 49) or Dorsal Root Ganglion Stimulation (DRG-S, n = 2) implantation due to intractable ischemic pain because of PVD (Rutherford 3, 4 and 5) from 03/2007 – 04/2022. Patients were classified as Rutherford’s class 3 (n = 21), class 4 (n = 12), or class 5 (n = 8)[RM(2] . Walking distance (m), pain intensity (NRS), opioid consumption (mg MME [morphine milligram equivalents]/d), and quality of life (EQ-5D VAS) were determined during follow-up. Two patients were excluded due to loss to follow-up within 6 months. Relevant comorbidities, major amputations, and deaths were documented. The Kaplan-Meier survival curve was used for the primary endpoint analysis for survival and amputation rates. Because of the observed mortality over the total period of eleven years, the 48-month follow-up was chosen as the cut-off time to ensure a sufficient sample size (IBM SPSS Statistics 29, Armonk, NY, USA).
Results
The number of patients with follow-up data at respective time points includes n = 49 patients at 6M, 41 at 1Y, 37 at 2Y, 27 at 3Y, 21 at 4Y, 16 at 5Y, 14 at 6Y, 13 at 7Y, 8 at 8Y and 1 patient at 9 and 10Y. We observed a statistically highly significant reduction in the level of pain at rest and likewise in load-dependent pain. 75% of patients were walking more than 200 m at 48M. 42/49 did not undergo a major amputation post-implant. We observed a significant reduction in opioid consumption and an improvement in the quality of life of treated patients.
Discussion
Neuromodulation reduces pain and improves the functionality of the affected limb and patient quality of life for patients suffering from severe PVD. Compared to existing literature (SCS can reduce the amputation rate and pain in selected patients over a period of 12 months2, 3) our data demonstrate a long-lasting therapeutic effect.
Conclusions
According to these results, neuromodulative therapy should be recommended for patients with non-reconstructable and non-unstable stage (Rutherford 3, 4, and 5) disease and shows favorable long-term results.
References
1Giannopoulos, S. & Armstrong, E. J. Medical therapy for cardiovascular and limb-related risk reduction in critical limb ischemia. Vascular Medicine 26, 210-224, doi:10.1177/1358863X20987612 (2021).Asimakidou, E. & Matis, G. K. Spinal cord stimulation in the treatment of peripheral vascular disease: a systematic review – revival of a promising therapeutic option? Brit J Neurosurg, 1-9, doi:10.1080/02688697.2021.1884189 PMID - 33703962 (2021).
2Asimakidou, E. & Matis, G. K. Spinal cord stimulation in the treatment of peripheral vascular disease: a systematic review – revival of a promising therapeutic option? Brit J Neurosurg, 1-9, doi:10.1080/02688697.2021.1884189 PMID - 33703962 (2021).
3Cucuruz, B. et al. Treatment of end-stage peripheral artery disease by neuromodulation. Clinical hemorheology and microcirculation 81, 315-324, doi:10.3233/ch-221436 (2022).
Learning Objectives
1. Spinal cord stimulation (SCS) can improve pain relief, limb salvage, and microcirculatory blood flow in patients suffering from intractable ischemic pain due to peripheral vascular disease (PVD).
2. This form of therapy shows favorable long-term efficacy, is safe and has only minimal side effects.
3. According to these results, neuromodulative therapy should be recommended for patients with non-reconstructable and non-unstable stage (Rutherford 3, 4, and 5) disease.
O024 - DEVELOPING AN AUGMENTATION APPROACH FOR ITBS IN MAJOR DEPRESSIVE DISORDER USING SYNCHRONISED TACS (ID 106)
Abstract
Introduction
Transcranial magnetic stimulation (TMS) is approved for treating major-depressive-disorder (MDD), a condition that affects 1 in 7 people during their lifetime1. An increasingly popular protocol, due to short session time, is intermittent theta burst stimulation (iTBS), in which TMS pulse triplets repeat at 5Hz (a “theta” frequency)2. Response to TMS, including iTBS, is highly variable3,4. We are examining a relatively inexpensive, easy-to-implement, approach to make iTBS act faster, for more people, by modifying “brain state” at the time of stimulation.
Materials / Methods
One aspect of “brain state” is oscillatory activity, generated by neural-circuit dynamics. Oscillatory activity mediates excitability within brain areas and connectivity between areas5. Pre-treatment fronto-central theta oscillatory power, measured with electroencephalography (EEG), is correlated with response to antidepressant medications6. Li et al.7 found that performance of a theta-eliciting task prior to TMS was associated with better clinical response, and post-task theta predicted response. However, task-based augmentation is unsuitable for people with more severe depression, characterised by cognitive and motivational difficulties8,9.
Our approach seeks to enhance theta activity during and after iTBS (and thus augment iTBS efficacy) using concurrent, synchronised, transcranial alternating-current stimulation (tACS). (Building on work showing combined tACS-TMS enhances oscillatory power for traditional “rTMS” protocols10). tACS is delivered at a theta-frequency (5Hz), with 1mA peak-to-peak amplitude, using 9cm2 round-electrodes at F3 (anode) and TP9 (cathode). iTBS at 80% resting-motor-threshold (20 cycles - 600 pulses, 3.3 minutes) is delivered via a 70mm figure-eight coil over F3, with pulse triplets timed to occur at the tACS peaks (Fig.1A).
Results
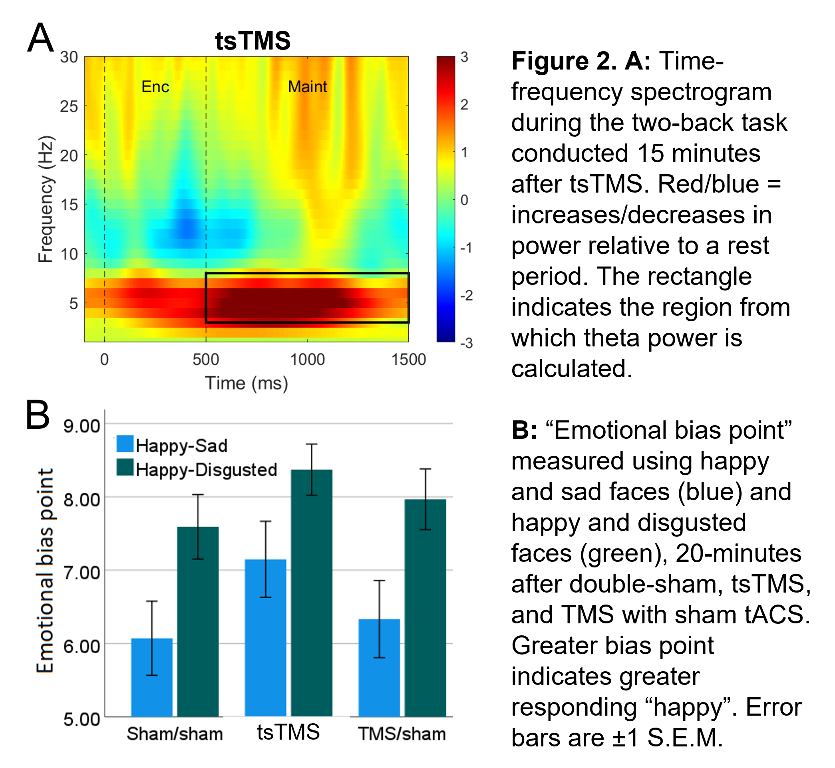
In an ongoing non-patient study (current N=12, 6 female, age±SD 24.1±5.6 years), theta activity during an n-back working-memory task is recorded before, and 1/8/15-minutes after, one session of “tACS-synchronised TMS” (tsTMS), TMS with sham-tACS, or double-sham. In the double-sham condition, theta decreased with repetition of the n-back task. In both stimulation conditions, this reduction was arrested, with an increase in theta (relative to pre-stimulation) at the 8/15-minute time points. At 15-minutes, theta was greatest in the tsTMS condition (Fig.1B,2A). At 20-minutes, positive emotional bias (a marker of potential antidepressant efficacy11,12, obtained from ratings of “happy” or “sad” to briefly-presented facial expressions) was greatest after tsTMS (followed by TMS alone, Fig.2B).
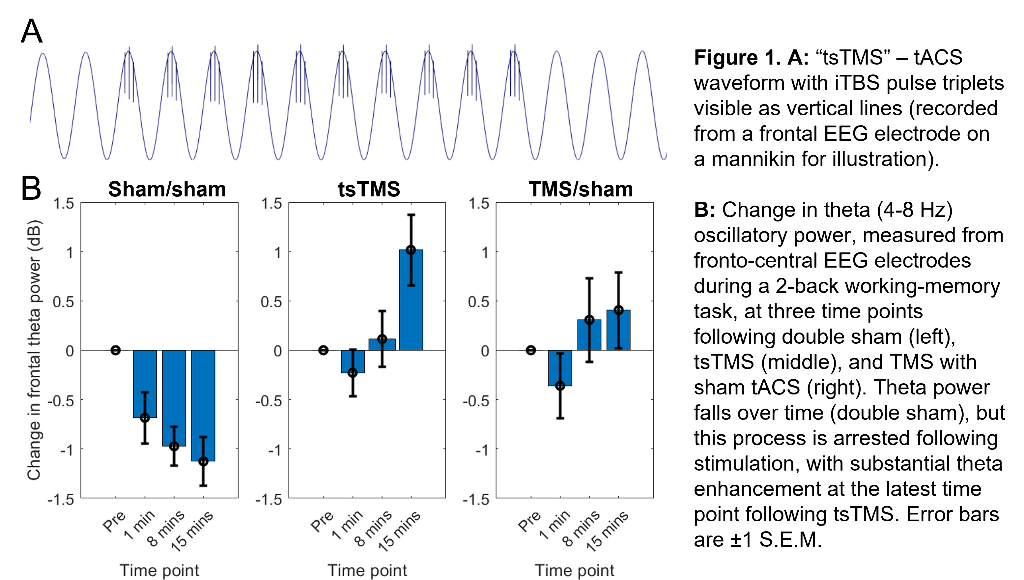
Discussion
The results suggest tACS-synchronisation may be able to augment the antidepressant effects of TMS. tsTMS was equally well-tolerated to TMS-alone.
Conclusions
Our next step is a pilot of multiple sessions of tsTMS with patients, to obtain further mechanistic evidence and estimates of clinical effect-size.
References
1Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:369-388. doi:10.1146/annurev-publhealth-031912-114409
2Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201-206. doi:10.1016/j.neuron.2004.12.033
3López-Alonso V, Cheeran B, Río-Rodríguez D, Fernández-Del-Olmo M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014;7(3):372-380. doi:10.1016/j.brs.2014.02.004
4Kaster TS, Downar J, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, Knyahnytska Y, Kennedy SH, Lam RW, Daskalakis ZJ, Blumberger DM. Trajectories of response to dorsolateral prefrontal rTMS in major depression: A three-D study. Am J Psychiatry. 2019;176(5):367-375. doi:10.1176/appi.ajp.2018.18091096
5To WT, de Ridder D, Hart J, Vanneste S. Changing brain networks through non-invasive neuromodulation. Front Hum Neurosci. 2018;12:128. doi:10.3389/fnhum.2018.00128
6Spronk D, Arns M, Barnett KJ, Cooper NJ, Gordon E. An investigation of EEG, genetic and cognitive markers of treatment response to antidepressant medication in patients with major depressive disorder: A pilot study. J Affect Disord. 2011;128(1-2):41-48. doi:10.1016/j.jad.2010.06.021
7Li CT, Hsieh JC, Huang HH, et al. Cognition-modulated frontal activity in prediction and augmentation of antidepressant efficacy: a randomized controlled pilot study. Cereb Cortex. 2016;26(1). doi:10.1093/cercor/bhu191
8Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200-206. doi:10.1192/bjp.178.3.200
9McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119:1-8. doi:10.1016/j.jad.2009.04.022
10Hosseinian T, Yavari F, Biagi MC, et al. External induction and stabilization of brain oscillations in the human. Brain Stimul. 2021;14(3):579-587. doi:10.1016/j.brs.2021.03.011
11Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59(9):816-820. doi:10.1016/j.biopsych.2005.10.015
12Harmer CJ, Cowen PJ. “It's the way that you look at it”—a cognitive neuropsychological account of SSRI action in depression. Philos Trans R Soc B: Biol Sci. 2013;368:201204073. doi:10.1098/rstb.2012.0407.
Learning Objectives
1. Gain awareness of current use of TMS in major depressive disorder
2. Understand how tACS-synchronisation can be achieved alongside TMS ("tsTMS")
3. Understand how tsTMS modifies markers of potential antidepressant efficacy (oscillatory activity, emotional bias)
PATIENT SELECTION FOR SPINAL CORD STIMULATION IN TREATMENT OF PAIN: SEQUENTIAL DECISION-MAKING MODEL — A NARRATIVE REVIEW (ID 111)
Abstract
Introduction
Despite the well-known efficacy of spinal cord stimulation (SCS) in chronic pain management, patient selection in clinical practice remains challenging. The aim of this review is to provide an overview of the factors that can influence the process of patient selection for SCS treatment.
Materials / Methods
A sequential decision-making model is presented within a tier system that operates in clinical practice.
Results
The first level incorporates the underlying disease as a primary indication for SCS, country-related reimbursement rules, and SCS screening–trial criteria in combination with underlying psychological factors as initial selection criteria in evaluating patient eligibility for SCS. The second tier is aligned with the individualized approach within precision pain medicine, whereby individual goals and expectations and the potential need for preoperative optimizations are emphasized. Additionally, this tier relies on results from prediction models to provide an estimate of the efficacy of SCS in the long term. In the third tier, selection bias, MRI compatibility, and ethical beliefs are included, together with recent technological innovations, superiority of specific stimulation paradigms, and new feedback systems that could indirectly influence the decision-making of the physician.
Discussion
This tier system can be considered a sequential decision-making model operating in daily routine care; however, often only the first and sometimes the second tier are openly discussed with the patient. In light of a free, fully informed decision-making process, patients should be fully informed about each tier in order to make an independent decision on whether or not to be considered adequate candidates for SCS and to initiate a treatment trajectory.
Conclusions
Both patients and physicians should be aware of the different aspects that influence patient selection in relation to SCS for pain management to make an independent decision on whether or not to initiate a treatment trajectory with SCS.
References
None.
Learning Objectives
1. To learn about individualised pain medicine in relation to a trajectory with neuromodulation.
2. Be aware of the ethical considerations and bias that is present during patient selection.
3. To gain insight in a sequential decision-making model to improve patient selection with transparancy for both patients and clinicians.
O017 - A STUDY ON THE ADVERSE EFFECTS OF TRANSCUTANEOUS SPINAL DIRECT CURRENT STIMULATION IN HEALTHY VOLUNTEERS (ID 130)
Abstract
Introduction
Since the first report of transcutaneous spinal direct current stimulation (tsDCS) on humans in 20081, more than 50 experimental studies have been published. However, systematic reports of adverse effects (AEs) and unspecific effects (UEs) of tsDCS are scarce. In this study, we aimed to systematically record tsDCS AEs via a structured questionnaire and also record UEs via tsDCS – concurrent monitoring of skin conductance, electrocardiographic and respiratory activity.
Materials / Methods
Twenty healthy participants (10 females, 20-40 years old) were recruited for this study. All of them underwent three consecutive sessions (at least 1 week apart) with active (anodal/cathodal) or sham stimulation in double-blinded way. A pair of rubber-electrodes was placed over the twelfth thoracic vertebra (anode) and the suprascapular region (cathode). The active stimulation was applied at 2.5 mA for 20 min with 15 sec for fade-in/fade-out, the sham stimulation only lasted for 45 sec with same intensity and fade-in/fade-out setting. Spontaneous skin conductance responses (SCR) were recorded from the right hand and electrocardiographic (ECG: heart-rate and its variability) and respiratory activity (breathing-rate and its variability) was measured from the chest. A tsDCS adverse effects questionnaire (Figure 1, adapted from Brunoni and colleagues2) was filled in by participants directly after tsDCS termination.

Results
Several potential AEs and whether they were deemed to be associated with tsDCS were recorded (Figure 2). Skin redness (60.66%), burning (40%), tinging (26.67%), and itching (20%) were the most reported AEs (with skin redness being reported by the experimenter). Most reported AEs occurred within the first minute of tsDCS onset, lasted for 1-2 minutes and solely occurred at the skin area beneath the electrodes. In some sessions, sleepiness (6.67%) and trouble concentrating (1.67%) were reported, but instead of being strongly associated with tsDSC, they were likely due to no task during tsDCS according to the participants' feedback. For all the AEs separately reported by participants, as well as their aggregation into one overall score, Bayesian Wilcoxon Signed-rank tests were employed to compare active (anodal or cathodal) and sham tsDCS. These were more indicative for an absence of differences between active and sham conditions (Figure 3, not a single Bayes Factor [BF] above 3, with several below 1/3 [thus providing moderate evidence against differences]). Similar results were obtained for UEs (Figure 4), which were analysed with a Bayesian repeated-measures ANOVA: no BF was above 3, with nearly all BFs being below 1 and often close to 1/3.


Discussion
Skin-related changes at the site of electrode contact were the main reported AEs. Bayesian testing provided moderate evidence for an absence of differences in several AEs and in no case was there evidence for a difference. Such a pattern of results is reassuring from a safety and blinding perspective, as it indicates that both active and sham interventions have a similar profile in terms of AEs. Somewhat weaker, but qualitatively similar results for autonomic responses between active and sham tsDCS suggests it possibly has minimal impact on the autonomic nervous system. However, further research is needed to validate these results in larger samples as well as for other locations (e.g. cervical tsDCS) and explore potential individual variations that may influence tsDCS related AEs.
Conclusions
In this pioneering study, we present a systematic recording of AEs and UEs associated with tsDCS in healthy participants. As research in this area expands, there is an increasing demand for safety-focused investigations, and our study offers the first comprehensive assessment, contributing valuable insights to the field.
References
1. Cogiamanian F, Vergari M, Pulecchi F, Marceglia S, Priori A. Effect of spinal transcutaneous direct current stimulation on somatosensory evoked potentials in humans. Clin Neurophysiol. 2008;119(11):2636-2640.
2. Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol. 2011;14(8):1133-1145.
Learning Objectives
1. Discuss with colleagues and experts in non-invasive neuromodulation field and look forward to geeting constructive advice.
2. Practice my presenting skills to clearly convey the idea of my research.
3. Establish a professional network and look forward to future collaborations that can be initiated.
O014 - SPINAL CORD STIMULATION (SCS) COMBINED WITH MUSCLE STIMULATION FOR THE TREATMENT OF CHRONIC BACK PAIN: MUSCLESCS TECHNIQUE: CLINICAL STUDY USING BURST SCS AS DEFINED BY DE RIDDER AND PERCUTANEOUS PLATE ELECTRODES (ID 140)
Abstract
Introduction
In a pilot study we showed that it is possible to generate pleasant and pain relieving muscle stimulation in the back by using low frequency SCS stimulation (MuscleSCS). In this following study we wanted to examine whether this technique can improve the treatment of lower back pain.
Materials / Methods
Patients with chronic low back pain had an SCS system (lamitrode) implanted after a trial phase and were then randomly treated with only Burst SCS as defined by De Ridder stimulation, only muscle stimulation or Burst SCS as defined by De Ridder and muscle stimulation combined for 2 weeks respectively.
Thereafter, the patients were treated for another 4.5 months with one of the 3 methods (cross-over possible). Pain ratings (Visual Analogue Scale VAS) were recorded daily, and Questionnaires (Pain Disability Index, PDI; Pain Catastrophizing Scale, PCS; Brief Pain Inventory, BPI) were used at baseline, at 3 and at 6 months.
Results
This is a prospective, multicentric, single-blinded, randomized crossover study. We included 58 patients (9 dropouts) (25 females, mean age 62.3 yrs). All 3 stimulation methods, including muscle stimulation alone, showed a highly significant pain relief when compared to the baseline value (p=0.001). The combined application of Burst SCS as defined by De Ridder with muscle stimulation showed the best results (p=0.032) (Fig.1). PDI, PCS and BPI improved significantly during this treatment. No serious adverse events occurred during this study. 75.5% of the subjects experienced an improvement in their pain as a result of this muscle stimulation.

Figure 1:
A bar graph shows the mean pain scores during study phase 1. The combined use (BM) of Burst SCS as defined by De Ridder (B) and MuscleSCS (M) showed the best results.
Discussion
SCS can adequately treat back pain [1]. So far, however, it has not been possible to adequately treat muscle pain with SCS neuromodulation. However, with low-frequency stimulation of 2-8 Hertz it is possible to reach the muscles by directly stimulating the motor neurons in the anterior horn of the spinal cord. This type of stimulation triggers a massage-like effect in the patient's muscles, which most patients describe as pleasant and pain-relieving.
Conclusions
This study showed that the combined use of SCS and additional low frequency muscle stimulation (MuscleSCS) could significantly improve the outcome of patients suffering from chronic back pain.
References
Eckermann JM, Pilitsis JG, Vannaboutathong C, Wagner BJ, Province-Azalde R, Bendel MA. Systematic Literature Review of Spinal Cord Stimulation in Patients With Chronic Back Pain Without Prior Spine Neuromodulation. 2021 Aug 18
Learning Objectives
MuscleSCS can further improve the treatment of back pain.
