Welcome to the ESPNIC Xperience Programme Scheduling
The meeting will run on Central European Summer Time
BIG DATA IN PAEDIATRIC CARDIAC ARREST – TRICK OR TREAT?
RESEARCH MONITORING PRACTICES IN INTENSIVE CARE RESEARCH: A SURVEY OF CURRENT STATE AND ATTITUDES
Abstract
Background and Aims
In 2016, ICH-GCP standards recommended that the approach to monitoring a research project should be undertaken based on risk, however it is unknown whether this approach has been adopted in Australia and New Zealand (ANZ) throughout intensive care research.
This study aimed to: describe current research monitoring practices in academic-led clinical trials in the field of critical care research; describe the perceived barriers and enablers to undertaking research monitoring; and relate respondent characteristics to research monitoring practices.
Methods
Electronic survey distributed to investigators, research co-ordinators and other research staff currently undertaking and supporting academic-led clinical trials in the field of intensive care in ANZ.
Results
The 115 respondents work in research units associated with hospitals (100; 87%) and academic-led trials (100; 86%) and are experienced Research Coordinators (60; 52%) and Principal Investigators (23; 20%); 36 (31%) are primarily associated with paediatric research. 68 respondents co-ordinate academic trials; the remaining results pertain to this sub-sample. 79% develop monitoring plans, with 49% of these undertaking a risk assessment; the most common barrier reported was lack of expertise. 32% indicated that centralised monitoring was used, noting that technology to support centralised monitoring (88%) along with support from data managers and statisticians (86%) were key enablers. COVID-19 impacted monitoring for 83% by increasing remote (55%) and reducing onsite (66%) monitoring.
Conclusions
Contrary to ICH-GCP guidance, risk assessments to inform monitoring plans are not being consistently performed due to lack of experience and guidance. There is an urgent need to enhance risk assessment methodologies and develop technological solutions for centralised statistical monitoring.
TOWARDS A EUROPEAN NICU AND PICU DATA SHARING FRAMEWORK: AN ESPNIC INITIATIVE
Abstract
Background and Aims
A framework to safely share clinical data from European Pediatric and Neonatal Intensive Care Units (PICU, NICU) will enhance research, improve clinical benchmarking and promote quality improvement projects among participating centres. When applying advanced data science methods such as machine learning to heterogenous patient data, substantial amounts of patient data from different centres may be needed to produce and validate reliable models.
The aim of this research is to summarize conditions and requirements which such a framework must meet in order to take the first steps towards a European NICU and PICU clinical data framework.
Methods
A survey was conducted among the members of the ESPNIC Data Science Working Group to investigate the use of electronic medical records (EMR) among European NICU’s and PICU’s and to identify European experts in NICU and PICU data registries and data science. During three meetings, these experts defined a list of topics regarding the framework conditions and requirements.
Results
The use of EMR ranged from approximately 20% in Germany to 100% in The Netherlands (Figure 1). Fourteen European experts in data registries and data science were identified. They reached consensus on the requirements for a European NICU and PICU data framework, which included data availability, data standardization, legislation and privacy, funding and framework architecture.
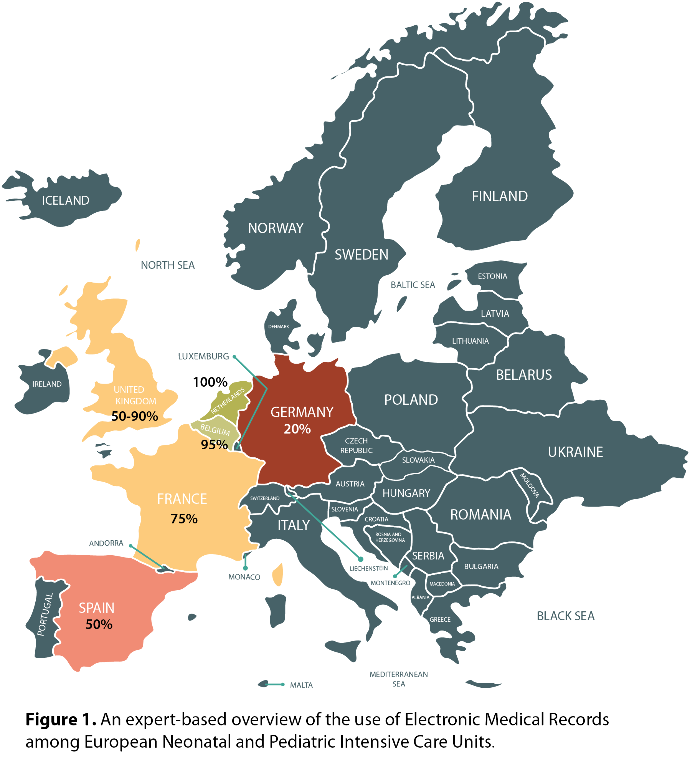
Conclusions
Several requirements for a European NICU and PICU clinical data sharing framework were identified, including data availability. The variable use of EMR among European centres may detain development of such a framework.
NON-INVASIVE ELECTRICAL BIOIMPEDANCE-BASED CARDIAC OUTPUT MONITORING IN NEONATES AND CHILDREN COMPARED TO STANDARD METHODS: A SYSTEMATIC REVIEW AND META-ANALYSIS.
Abstract
Background and Aims
This study aimed to systematically review and meta-analyze the validity of electrical bioimpedance-based non-invasive cardiac output (CO) monitoring in paediatrics compared to standard methods such as thermodilution and echocardiography.
Methods
PRISMA guidelines were followed. MEDLINE and EMBASE were systematically searched (2000-2019) for method-comparison studies comparing transthoracic or whole body electrical bioimpedance devices to standard methods in children (0-18 years old). Following study selection, means of CO, stroke volume (SV) and cardiac index (CI) measurements by compared devices were extracted, as were Bland Altman analysis results. Mean differences were pooled using a random-effects model, R Core Team, 2019.
Results
29 of 649 identified studies were included. There was no significant difference between means of CO, SV and CI measurements, except in the neonatal/infant studies reporting SV (mean difference 1.00ml, 95%CI 0.23–1.77). Median percentage error (PE) was close to acceptability (PE ≤30%) in child/adolescent studies for CO in L/min (31%) and SV in ml (26%), but not in neonatal/infant studies (45% for CO and SV). PE was >30% in six of nine studies with heterogeneous congenital heart disease populations. Subgroup analysis revealed artificial ventilation did not significantly affect pooled mean difference in child/adolescent CO and SV studies (Figure 1 shows the subgroup analysis for CO, L/min).
Conclusions
Studies included used heterogeneous designs, devices and populations; only one study included haemodynamically unstable patients. Bioimpedance-based devices may be valid for use in paediatrics, but validity remains uncertain in neonates, congenital heart disease, and cardiovascularly unstable patients. Larger studies in specific clinical contexts with standardised methodologies are required.
