Welcome to the e-INS 2023 Interactive Program
123 Presentations
O003 - CLOSED-LOOP SCS FOR THE TREATMENT OF CHRONIC PAIN ASSOCIATED WITH RAYNAUD’S PHENOMENON (ID 144)
Abstract
Introduction
Raynaud's phenomenon (RP) is an episodic vasospasm of the peripheral arteries that causes cyanosis, erythema, pain, paraesthesia’s, and sometimes ulceration of the fingers and/or toes1. There are few reports, mostly case series, on the benefits of spinal cord stimulation (SCS) for the treatment of RP2–19. However, there is a lack of objective evidence on SCS induced modulation of the sympathetic system (e.g., vasodilation) in this condition.
We hypothesize that evoked compound action potential-(ECAP)-controlled closed-loop-SCS may relieve pain and reduce the severity and frequency of Raynaud's attacks. Furthermore, we hypothesize that the retrograde effects of ECAP-controlled closed-loop-SCS may improve peripheral blood flow. Here, objective results on the effects on peripheral circulation and subjective changes in the frequency and severity of Raynaud's attacks will be presented. Full cohort data will be introduced at eINS.
Materials / Methods
This is a prospective, single-centre pilot study to evaluate the efficacy of ECAP-controlled closed-loop-SCS (Evoke® SmartSCSTM, Saluda Medical, Australia) in the treatment of RP. Patient outcomes such as Raynaud severity/condition score, Cochin-Hand-Function-Scale, SHAQ-RP-VAS, EQ-5D-5L, PGIC, stimulation parameters, objective peripheral blood flow assessments and neurophysiological measurements were collected at baseline, trial end, 1-month, 3-months, and 6-months.
Results
The mean age ±standard deviation (±SD) patients was 45.5±15.5 years (n=10), and 80% were female.
Fig.1A shows an occluded distal digital artery due to RP, whereas Fig.1B shows the same section after 3-months of SCS, revealing blood flow restoration at the level of the Distal interphalangeal joint.
Pathological arterial occlusions decreased by 49% (n=8; Fig.1C) at 6-months after implantation.
Recording and measurement of patients' ECAPs in the clinic are presented in Fig.1D. The Voltage (µV) increased with increasing current.

The mean baseline (±SEM; n=9) Raynaud's condition score was 6.6±0.7 and decreased to 2.6±0.7 6-months after implantation (n=8; Fig.2A). Patients experienced a clinically meaningful change in symptom severity as informed by the Raynaud's condition score (-1.4)20.
The mean baseline (±SEM; n=9) Cochin-Hand-Function-Scale score was 31.6±8.2 and decreased to 17.4±7.7 6-months after implantation (n=8; Fig.2B).

Discussion
This study demonstrates for the first time that RP-related pathological arterial occlusions and Raynaud symptoms can be treated with a novel ECAP-controlled closed-loop-SCS system.
Conclusions
In conclusion, ECAP-controlled closed-loop-SCS alleviates RP symptoms and improves peripheral blood flow. Longer-term follow up and larger controlled studies are needed to confirm these preliminary pilot study results.
References
1. Herrick, A.L. (2012). The pathogenesis, diagnosis and treatment of Raynaud phenomenon. Nat. Rev. Rheumatol. 8, 469–479. https://doi.org/10.1038/nrrheum.2012.96.
2. Barba, A., Escribano, J., and García-Alfageme, A. (1992). The treatment of vasospastic disease by chronic spinal cord stimulation. A case report. Angiologia 44, 136–138.
3. Benyamin, R., Kramer, J., and Vallejo, R. (2007). A Case of Spinal Cord Stimulation in Raynaud’s Phenomenon: Can Subthreshold Sensory Stimulation Have an Effect? Pain Physician, 6.
4. Chapman, K.B., Kloosterman, J., Schor, J.A., Girardi, G.E., van Helmond, N., and Yousef, T.A. (2021). Objective improvements in peripheral arterial disease from dorsal root ganglion stimulation: a case series. Ann. Vasc. Surg. 74, 519-e7.
5. Devulder, J., De Colvenaer, L., Rolly, G., Caemaert, J., Calliauw, L., and Martens, F. (1990). Spinal cord stimulation in chronic pain therapy. Clin. J. Pain 6, 51–56.
6. ERTILAV, E., and AYDIN, O.N. (2020). Spinal cord stimulator for the treatment of ischemic pain-Burger’s Disease and Raynaud’s disease: A report of 2 cases and literature.
7. Francaviglia, N., Silvestro, C., Maiello, M., Bragazzi, R., and Bernucci, C. (1994). Spinal cord stimulation for the treatment of progressive systemic sclerosis and Raynaud’s syndrome. Br. J. Neurosurg. 8, 567–571.
8. Giglio, M., Preziosa, A., Rekatsina, M., Viswanath, O., Urits, I., Varrassi, G., Paladini, A., and Puntillo, F. (2021). Successful spinal cord stimulation for necrotizing Raynaud’s phenomenon in COVID-19 affected patient: the nightmare comes back. Cureus 13.
9. Issa, M.A. (2012). Cervical Spinal Cord Stimulation with 5-ColumnPaddle Lead In Raynaud’s Disease. Pain Physician 4;15, 303–309. 10.36076/ppj.2012/15/303.
10. Ito, H., Tanei, T., Sugawara, K., Sando, Y., and Hori, N. (2022). Spinal cord stimulation for the treatment of pain and toe ulceration associated with systemic sclerosis: a case report. FUKUSHIMA J. Med. Sci. 68, 37–41. 10.5387/fms.2021-33.
11. Münster, T., Tiebel, N., Seyer, H., and Maihöfner, C. (2012). Modulation of Somatosensory Profiles by Spinal Cord Stimulation in Primary Raynaud′s Syndrome: SCS Influences QST in Primary Raynaud′s Syndrome. Pain Pract. 12, 469–475. 10.1111/j.1533-2500.2012.00531.x.
12. Neuhauser, B., Perkmann, R., Klinger, P.J., Giacomuzzi, S., Kofler, A., and Fraedrich, G. (2001). Clinical and Objective Data of Spinal Cord Stimulation for the Treatment of Severe Raynaud’s Phenomenon. EJVES Extra 1, 3–4. 10.1053/ejvx.2000.0002.
13. Niclauss, L., Roumy, A., and Gersbach, P. (2013). Spinal Cord Stimulation in Thromboangiitis Obliterans and Secondary Raynaud’s-Syndrome. EJVES Extra 26, e9–e11. 10.1016/j.ejvsextra.2013.03.007.
14. Provenzano, D.A., Nicholson, L., Jarzabek, G., Lutton, E., Catalane, D.B., and Mackin, E. (2011). Spinal Cord Stimulation Utilization to Treat the Microcirculatory Vascular Insufficiency and Ulcers Associated with Scleroderma: A Case Report and Review of the Literature. Pain Med. 12, 1331–1335. 10.1111/j.1526-4637.2011.01214.x.
15. Robaina, F.J., Dominguez, M., Díaz, M., Rodriguez, J.L., and de Vera, J.A. (1989). Spinal cord stimulation for relief of chronic pain in vasospastic disorders of the upper limbs. Neurosurgery 24, 63–67.
16. Sciacca, V., Petrakis, I., and Borzomati, V. (1998). Spinal cord stimulation in vibration white finger. VASA Z. Gefasskrankheiten 27, 247–249.
17. Sibell, D.M., and Stacey, B.R. (2005). Successful Use of Spinal Cord Stimulation in the Treatment of Severe Raynaud’s Disease of the Hands. CASE Rep. 102, 5.
18. Ting, J.C., Fukshansky, M., and Burton, A.W. (2007). Treatment of Refractory Ischemic Pain from Chemotherapy-Induced Raynaud?s Syndrome With Spinal Cord Stimulation. Pain Pract. 7, 143–146. 10.1111/j.1533-2500.2007.00122.x.
19. Wolter, T., and Kieselbach, K. (2011). Spinal Cord Stimulation for Raynaud’s Syndrome: Long-Term Alleviation of Bilateral Pain With a Single Cervical Lead: SPINAL CORD STIMULATION FOR RAYNAUD’S SYNDROME. Neuromodulation Technol. Neural Interface 14, 229–234. 10.1111/j.1525-1403.2011.00332.x.
20. Khanna, P.P., Maranian, P., Gregory, J., and Khanna, D. (2010). The minimally important difference and patient acceptable symptom state for the Raynaud’s condition score in patients with Raynaud’s phenomenon in a large randomised controlled clinical trial. Ann. Rheum. Dis. 69, 588–591. 10.1136/ard.2009.107706.
Learning Objectives
1. To learn that pathological arterial occlusions in patients with Raynaud’s Phenomenon can be treated with ECAP-controlled closed-loop-SCS.
2. To show that Raynaud symptoms can be treated with ECAP-controlled closed-loop-SCS.
3. To provide that overall assessment of an SCS therapy for chronic neuropathic pain in Raynaud’s Phenomenon requires the implementation of objective and multiple patient-related outcomes measures.
O002 - ECAP-CONTROLLED CLOSED-LOOP SCS IN A REAL-WORLD SETTING IN EUROPE (ID 152)
Abstract
Introduction
Evoked compound action potential (ECAP)-controlled closed-loop spinal cord stimulation (SCS) provides superior pain relief compared to traditional ‘open-loop’ SCS due to its ability to maintain consistent and accurate activation of the spinal cord [1],[2]. Results from controlled studies are sometimes hard to repeat in the real-world. Here, interim real-world results of the multi-center data collection study are presented from 13 centers across Europe.
Materials / Methods
This multi-center data collection (Clinical Trials registry ID: NCT05272137) was designed to collect patient reported outcomes for pain relief, Verbal Numerical Rating Scale (VNRS) and satisfaction (five options, ranging from “Very Satisfied” to “Very Unsatisfied”). Additionally, electrophysiological data (ECAPs) and device data (stimulation parameters, patient usage) were collected during standard-of-care visits for patients treated with ECAP-controlled closed-loop SCS system (Evoke® SmartSCSTM, Saluda Medical, Australia) in a real-world setting under normal clinical use in Europe.
Interim results from this study are presented below. Patients presenting with CRPS Type 1 and 2, PSPS type 2 and polyneuropathy, post amputation stump pain and peripheral plexopathy were enrolled. Post-market visit requirements followed standard of care; if a follow-up visit was not performed, or patient outcomes were not taken due to time limitations, it was not regarded as a protocol deviation.
Results
A total of 153 patients underwent permanent implantation of the ECAP-controlled closed-loop system. Mean (±SEM) baseline (n=153) VNRS scores were 8.12±0.10. At 3-months (n=103) average VNRS scores decreased to 2.43±0.22, at 6-months (n=83) to 2.51±0.26, at 12-months (n=74) to 2.50±0.26 and at 24-months to 2.46±0.40 (n=24; Fig.1A).
At 12-months there were 80% responders (≥50% pain relief), and 43% high-responders (≥80% pain relief; Fig.1B).
At 12-months, 92% of the patients reported being very satisfied or satisfied with their therapy (Fig.1C; n=68 of 74 patients). Patients used closed-loop SCS at 12-months (n=48) above perception threshold (Neural Activation Level; Mode ECAP: 9.8µV) identified in-clinic (Fig.1D).

Discussion
Results strongly suggest that ECAP-controlled closed-loop SCS can lead to a high degree of pain relief and patient satisfaction 12-months post-implantation in a real-world setting. The final results will be presented at the eINS conference.
Conclusions
Preliminary real-world results are comparable to results from the controlled AVALON multi-center-study [3] and the controlled EVOKE RCT [1] in pain relief outcomes.
References
[1] N. Mekhail et al., “Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial,” Lancet Neurol., p. S1474442219304144, Dec. 2019, doi: 10.1016/S1474-4422(19)30414-4.
[2] N. Mekhail et al., “Durability of Clinical and Quality-of-Life Outcomes of Closed-Loop Spinal Cord Stimulation for Chronic Back and Leg Pain: A Secondary Analysis of the Evoke Randomized Clinical Trial,” JAMA Neurol., vol. 79, no. 3, pp. 251–260, Jan. 2022, doi: 10.1001/jamaneurol.2021.4998.
[3] M. Russo et al., “Sustained Long-Term Outcomes With Closed-Loop Spinal Cord Stimulation: 12-Month Results of the Prospective, Multicenter, Open-Label Avalon Study,” Neurosurgery, Feb. 2020, doi: 10.1093/neuros/nyaa003.
[4] C. Brooker et al., “ECAP‐Controlled Closed‐Loop Spinal Cord Stimulation Efficacy and Opioid Reduction Over 24‐Months: Final Results of the Prospective, Multicenter, Open‐Label Avalon Study,” Pain Pract., 2021.
Learning Objectives
1. To learn that ECAPs are used to adjust stimulation levels in real-time to maintain consistent activation of the spinal cord.
2. To provide that ECAP-controlled closed-loop SCS in the real-world can lead to a high degree of pain relief and patient satisfaction 12-months post-implantation.
3. To provide evidence that real-world results are comparable to results from the AVALON multi-center-study and the EVOKE RCT in pain relief outcomes.
O026 - TRANSCRANIAL DIRECT CURRENT STIMULATION (TDCS) MODULATES CORTICAL NETWORKS DURING VISIOMOTOR LEARNING IN SCHIZOPHRENIA (ID 155)
Abstract
Introduction
Transcranial Direct Current Stimulation (tDCS) is a non-invasive brain stimulation approach in which low level currents are applied across the scalp to influence brain function (rev. in1). tDCS and other non-invasive brain stimulation approaches have the potential to increase plasticity and enhance cortical function2, enhance neurorehabilitation3, and reverse deficits in neuropsychiatric disorders such as schizophrenia4. While most studies have focused on its effects on local excitability (for discussion see5), more recent studies have focused on network-level effects across cortical regions6. Here, we used the high temporal resolution of EEG combined with a well-studied (rev. in7) visuo-motor learning paradigm – the serial reaction time task (SRTT) - to investigate cortical network mechanisms underlying tDCS effects in patients with schizophrenia.
Materials / Methods
Eighteen patients with schizophrenia and seventeen matched controls, aged 19–54, participated in the study. During the EEG, subjects performed the SRTT, consisting of presentation of a 5-element repeat sequence of color-coded visual cues, and were asked to press one of four color-coded keys as quickly as possible following presentation of each visual cue. During the EEG, subjects received four stimulation conditions on separate days (Sham, Motor- cathodal, Visual-cathodal, Motor-anodal) using a constant current of 2 mA intensity applied for 30 min during task performance.
Results
We analyzed mean RT as a function of condition across groups during stimulation and found a highly significant effect of Group, stimulation condition and Group X Condition interaction. In EEG, we used a source-space analysis approach to evaluate pairwise beta-frequency (12-18 Hz) coherence between dorsal-visual, SMA and motor nodes of the canonical SRTT circuit8,9 as a function both of motor learning and tDCS effect. In controls, motor-cathodal stimulation and visual-cathodal stimulation significantly modulated the coherence across motor cortex and SMA. In patients, motor-cathodal stimulation significantly modulated the coherence between SMA and visual cortex, visual-cathodal stimulation significantly modulated the coherence across motor cortex and SMA and between motor cortex and visual cortex.
Discussion
We observed robust effects when EEG responses were mapped into source space. In particular, beta-coherence measures were sensitive to the differential network modulatory effects of tDCS when comparing healthy controls and patients. In addition to its specific findings regarding tDCS modulation of motor learning, the present study may also have direct implications for use of network-targeted tDCS for treatment of motor manifestations of neuropsychiatric disorders in general.
Conclusions
We demonstrate that source-level beta-coherence measures obtained concurrent with tDCS may serve as effective target engagement biomarker for motor learning.
References
1- Brunoni, A. R. et al. Understanding tDCS effects in schizophrenia: a systematic review of clinical data and an integrated computation modeling analysis. Expert review of medical devices 11, 383-394, doi:10.1586/17434440.2014.911082 (2014).
2- Nissim, N. R. et al. Effects of Transcranial Direct Current Stimulation Paired With Cognitive Training on Functional Connectivity of the Working Memory Network in Older Adults. Frontiers in Aging Neuroscience 11, doi:10.3389/fnagi.2019.00340 (2019).
3- Sánchez-Kuhn, A., Pérez-Fernández, C., Cánovas, R., Flores, P. & Sánchez-Santed, F. Transcranial direct current stimulation as a motor neurorehabilitation tool: an empirical review. BioMedical Engineering OnLine 16, doi:10.1186/s12938-017-0361-8 (2017).
4- Brunelin, J. et al. Examining Transcranial Direct-Current Stimulation (tDCS) as a Treatment for Hallucinations in Schizophrenia. American Journal of Psychiatry 169, 719-724, doi:10.1176/appi.ajp.2012.11071091 (2012).
5- Polanía, R., Nitsche, M. A. & Ruff, C. C. Studying and modifying brain function with non-invasive brain stimulation. Nature Neuroscience 21, 174-187, doi:10.1038/s41593-017-0054-4 (2018).
6- Sehatpour, P. et al. Network-level mechanisms underlying effects of transcranial direct current stimulation (tDCS) on visuomotor learning. NeuroImage, 117311, doi:10.1016/j.neuroimage.2020.117311 (2020).
7- Buch, E. R. et al. Effects of tDCS on motor learning and memory formation: A consensus and critical position paper. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 128, 589-603, doi:10.1016/j.clinph.2017.01.004 (2017).
8- Keele, S. W., Ivry, R., Mayr, U., Hazeltine, E. & Heuer, H. The cognitive and neural architecture of sequence representation. 110, 316-339, doi:10.1037/0033-295x.110.2.316 (2003).
9- Kantak, S. S., Mummidisetty, C. K. & Stinear, J. W. Primary motor and premotor cortex in implicit sequence learning--evidence for competition between implicit and explicit human motor memory systems. The European journal of neuroscience 36, 2710-2715, doi:10.1111/j.1460-9568.2012.08175.x (2012).
Learning Objectives
1- To characterize the underlying network-level mechanisms of action of tDCS in improving motor learning in schizophrenia and related neuropsychiatric disorders.
2- To characterize the differential network effects of tDCS between healthy controls and patients with schizophrenia.
3- To investigate the cortical network effects of tDCS as potential target engagement biomarkers during clinical treatment studies.
O011 - PAIN THERAPY WITH SPINAL CORD STIMULATION (SCS) IN PATIENTS WITH PAINFUL DIABETIC NEUROPATHY (PDN): RESULTS OF A BUDGET IMPACT MODEL BASED ON GERMAN HEALTH-CLAIMS DATA (ID 164)
Abstract
Introduction
Diabetic polyneuropathy (DPN) is one of the most common complications occurring in patients with diabetes mellitus (DM). DPN manifests as sensory disturbance in the limbs and can be divided into painful/non-painful DPN. Due to complications associated with DPN, this disease could lead to high health care utilization. We aimed to analyse the cost structure and resource use in patients suffering from painful/non-painful DPN from the perspective of the German statutory health insurances (SHI) with the target creating a budget impact model (BIM) for the use of spinal cord stimulation (SCS).
Materials / Methods
The BIM was built using data from anonymized, age- and sex-adjusted routine data from 2014-2019 on approximately 4.9 million SHI-insured persons. With corresponding ICD- and ATC-codes, patients with DM were selected, stratified into patients with/without DPN and thereafter differentiated between painful/non-painful DPN. Cost analysis was based on mean annual costs per patient. For the BIM, we assumed a SCS therapeutic success of 85% (following Peterson et al. 2021) and for calculation of follow-up costs, an inflation rate of 2% was used. Costs (per patient) of painful DPN-patients with/without SCS were compared over the follow-up period (5 years).
Results
Patients with painful DPN showed total annual costs of 22,266€ compared to 9,727€ for patients with non-painful DPN, whereby the highest costs occurred in the inpatient sector. Sick pay and work incapacity days also increased especially with the presence of painful DPN. The BIM showed cost savings in SCS-treated patients with painful DPN from the 3rd year onwards (year 3: 4,122€ cost savings per patient).
Discussion
The results show that patients with painful DPN cause 2.3 times higher total annual costs for SHI compared to patients with non-painful DPN. Treatment options like SCS could help to relieve pain significantly and to save costs in those patients over the time.
Conclusions
From a payers’ perspective, patients with painful DPN are cost intensive and requiring special attention. Thus, care management and therapies increasing efficiency in order to reduce average costs are of particular interest in this area. Based on the BIM, SCS could play a significant role since it has the potential of saving costs from the third year after implantation.
References
Luecke, T., Siegel, E., Vogelmann, T.: „Kosten und Ressourcenverbrauch von Patient:innen mit schmerzhafter diabetischer Polyneuropathie“, in: „Monitor Versorgungsforschung“ (06/22), S. 61-67. http://doi.org/10.24945/MVF.06.22.1866-0533.2464.
Petersen EA, et al. (2021): Effect of High-frequency (10-kHz) Spinal Cord Stimulation in Patients With Painful Diabetic Neuropathy: A Randomized Clinical Trial. JAMA Neurol. 78(6):687-698.
Weinand, S., Luecke T., Siegel E., Vogelmann T.: „Pain Therapy With Spinal Cord Stimulation (SCS) in Patients With Painful Diabetic Neuropathy (PDN): Results of a Budget Impact Model”, in: Value in Health, Volume 25, Issue 12S (December 2022).
Learning Objectives
- Differentiation into cost drivers and impact of payer budgets
- Focus on increasing efficiency and cost savings
HOLISTIC TREATMENT RESPONSE WITH ECAP CLOSED-LOOP SPINAL CORD STIMULATION THERAPY: EVIDENCE FROM THE EVOKE RANDOMISED CONTROLLED TRIAL THROUGH 36-MONTHS (ID 165)
Abstract
Introduction
Chronic pain patients may experience impairments in multiple health-related domains. The design and interpretation of clinical trials of chronic pain interventions, however, remains primarily focused on treatment effects on pain intensity. This study investigates a novel, multidimensional holistic treatment response to ECAP-controlled closed-loop spinal cord stimulation (CL-SCS) versus open-loop SCS (OL-SCS) as well as the degree of neural activation that produced that treatment response through 36-month follow-up.
Materials / Methods
The EVOKE multicentre, double-blind, parallel-arm RCT was designed to evaluate the safety and efficacy of ECAP-based therapy to treat chronic back and leg pain (NCT02924129).1,2 Outcome data for pain intensity, physical function, health-related quality-of-life, sleep quality and emotional function were collected. Evaluation of holistic treatment response considered whether the baseline score was worse than normative values and whether minimal clinical important differences (MCIDs) were reached in each of the domains impaired at baseline.3 A cumulative responder score was calculated to reflect total MCIDs across all domains. Data analysis followed the intention-to-treat principle. Neural activation accuracy, defined as the deviation of the observed ECAP response from the prescribed ECAP was measured.
Results
Patients were randomised to CL-SCS (n=67) or OL-SCS (n=67). A greater proportion of patients with CL-SCS (44.8% vs 28.4%) were holistic responders at 36-month follow-up. The cumulative responder score was significantly greater for CL-SCS patients at 3-months (11.4 vs 8.7, MD=2.6, 95% CI=0.3-5.0, p=0.028), and resulted in more than 3 additional MCIDs at 12- (11.1 vs 7.7, MD=3.4, 95% CI=1.0-5.7, p=0.005), 24- (10.2 vs 6.8, MD=3.4, 95% CI=1.3-5.5, p=0.002) and 36-month follow-up (10.5 vs 7.2, MD=3.3, 95% CI=1.1-5.5, p=0.003). Neural activation was 3 times more accurate in CL-SCS (p<0.001 at all timepoints).


Discussion
Responders in multiple domains were observed as early as 3-months following SCS implantation and sustained through 36-months. This was particularly evident in the CL-SCS group which showed no degradation in responder rates across multiple domains. The superior patient-reported outcomes observed with CL-SCS show that ECAP-controlled therapy was delivered with greater accuracy and that this can provide a greater breadth and depth of improvements in multiple domains. This is unique in the current SCS literature.
Conclusions
The results of this study suggest that CL-SCS can result in true relief of the complex, multifactorial personal experience that patients describe as chronic pain. Additionally, with consistent neural activation at the prescribed level, CL-SCS provided superior and durable outcomes in all domains and at all timepoints when compared to OL-SCS.
References
1. Mekhail N, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol 2020; 19: 123-134.
2. Mekhail N, et al. Durability of Clinical and Quality-of-Life Outcomes of Closed-Loop Spinal Cord Stimulation for Chronic Back and Leg Pain: A Secondary Analysis of the Evoke Randomized Clinical Trial. JAMA Neurol 2022; 79: 251-260.
3. Levy RM, et al. Holistic Treatment Response: An International Expert Panel Definition and Criteria for a New Paradigm in the Assessment of Clinical Outcomes of Spinal Cord Stimulation. Neuromodulation 2023; Online ahead of print.
Learning Objectives
1. Evaluation of a holistic treatment response is paramount in chronic pain populations given that impairment can be present in several domains other than just pain intensity.
2. ECAP-controlled closed-loop SCS can provide a holistic response, characterised by improvement of at least one MCID in all measured health-related domains impaired at baseline.
3. ECAP-guided programming delivered for open-loop SCS and closed-loop SCS appear to provide clinical benefit.
O029 - THE UNTOLD STORY OF OCCIPITAL NERVE STIMULATION IN CLUSTER HEADACHE PATIENTS: SURGICAL TECHNIQUE IN RELATION TO CLINICAL EFFICACY (ID 168)
Abstract
Introduction
Cluster headache (CH) is described to be one of the most painful conditions known to humans and affects about one in every 1000 adults (1,2). CH is a primary headache disorder characterized by an excruciating unilateral temporal or periorbital pain, sometimes accompanied by autonomic symptoms (3). Though its exact pathophysiology remains unknown, disturbed nociceptive signaling within the occipital and trigeminal nerve circuits are considered major causes of CH (4). Occipital nerve stimulation (ONS) is recognized as a promising treatment for CH patients refractory to standard pharmacological approaches. Despite its effectiveness in many patients, adequate pain relief is not reached in a subset of CH patients undergoing ONS (5). Reasons for failure of ONS are wrong diagnosis, indication and anatomical variations combined with different surgical approaches. The current study provides a clear analysis including elaborate visualization on literature regarding the technique of ONS with regard to the anatomy. Additionally, cadaveric experimentation is combined with our clinical experience to provide a standardized proposal for ONS.
Materials / Methods
Data from 65 articles were analyzed. For the cadaveric experimentation (N=1), two electrodes were inserted in various directions from the region of C2 and foramen magnum and projected towards the mastoid process. Thereafter, the GON was dissected, and x-ray imaging was performed from different angles.
Results
Inconsistencies in anatomical location and landmarks of the occipital nerve were found in literature. Upon the current analysis, deviations in surgical aspects including electrode placement, imaging technique and patient positioning were also found, with a clinical efficacy ranging from 35-90%.


Discussion
The absence of a standardized protocol was confirmed in this analysis due to the existence of multiple approaches and variations in ONS. Deviations in surgical approaches are likely to be responsible for the broad efficacy range reported for ONS. Implementation of a universal approach to ONS can therefore improve management for all patients with refractory CH. Hence, we propose a standardized protocol for ONS. In this proposal, electrodes should be placed higher upon the occipital area. This approach is expected to hold great potential to optimize clinical benefit of ONS due to increased electric field coverage. Even though current observations are promising, follow-up studies are required to confirm the efficacy of the current proposal.
Conclusions
There is no standardized surgical protocol for ONS. The presence of such a protocol allows for better comparison of data and outcome between different institutions and physicians and is hence necessary to optimize treatment for CH.
References
1) Wei DY, Yuan Ong JJ, Goadsby PJ. Cluster Headache: Epidemiology, Pathophysiology, Clinical Features, and Diagnosis. Ann Indian Acad Neurol. 2018;21(Suppl 1):S3-s8.
2) Weaver-Agostoni J. Cluster headache. Am Fam Physician. 2013;88(2):122-8.
3) May A, Schwedt TJ, Magis D, Pozo-Rosich P, Evers S, Wang SJ. Cluster headache. Nat Rev Dis Primers. 2018;4:18006.
4) Eskilsson A, Ageberg E, Ericson H, Marklund N, Anderberg L. Decompression of the greater occipital nerve improves outcome in patients with chronic headache and neck pain—a retrospective cohort study. Acta Neurochirurgica. 2021;163(9):2425-33.
5) Eghtesadi M, Leroux E, Fournier-Gosselin M-P, Lespérance P, Marchand L, Pim H, et al. Neurostimulation for refractory cervicogenic headache: a three-year retrospective study. Neuromodulation: Technology at the Neural Interface. 2018;21(3):302-9.
Learning Objectives
1) There is a wide variety in the use of anatomical landmarks, patient positioning, imaging, electrode placement in the technique of occipital neurostimulation
2) There is a need for a standardized surgical protocol in occipital nerve stimulation
3) Such a protocol enables comparison of data between physicians who treat patients suffering from intractable cluster headache with occipital nerve stimulation
O032 - INTRATHECAL THERAPY (ITT) IN CHRONIC PAIN AND SEVERE SPASTICITY - A SINGLE-CENTER COHORT OF PATIENTS WITH LONG-TERM DRUG DELIVERY (ID 177)
Abstract
Introduction
Intrathecal drug delivery systems are approved with different analgesics in chronic refractory pain as well as baclofen in severe spasticity. In this retrospective analysis the clinical data of long-term ITT patients, which received pump exchanges between January 2008 and June 2020, were evaluated.
Materials / Methods
Overall, 116 pump devices were implanted in 71 patients (37 male, 34 female). ITT was used to treat chronic (non-malignant) pain in 36 patients and severe spasticity in 34 patients. In one patient, both indications coexisted. The follow-up period on average was 86 months (range: 12 to 147 months). 9 patients were excluded because of missing data. Any changes of intrathecal medication or application mode and the appearance of adverse events were collected.
Results
During the observation time, 30 patients received the first pump implantation and 41 patients had already been implanted before (27 battery-driven, 14 gas-driven devices). Pump exchanges were necessary in 56 patients for the first, in 15 patients for the second or more times. The most common cause for an exchange after a running period of approximately 6 years was battery depletion (n=51) of battery-driven pump devices.
Chronic pain was initially treated as intrathecal monotherapy with morphine in 26, ziconotide in six patients and hydromorphone in one patient. In three patients, morphine was combined with clonidine (n=2) or ziconotide (n=1) and in one case with baclofen for pain and spasticity treatment. All other patients (n=34) with spastic symptoms received baclofen monotherapy. After initial implantation a continuous application mode of the daily dosage was programmed. A flexible mode of drug delivery was used in three, a personal therapy manager in four patients. Adverse drug reactions were averted by dosage adjustments or a change of the intrathecal drug.
Complications were documented in 20 patients as follows: pump dysfunction/dislocation (n=18), spinal catheter disconnection/occlusion (n=14), wound healing disorder (n=5) or drug induced events (n=4).
Discussion
The long-term ITT was effective in controlling pain and spasticity, even over the lifetime of a pump device. Any exchanges were indicated, necessary and desired by the patients. The average time up to pump exchange is comparable to data from other publications. Occurrence of adverse events is usually managed by dosage adjustments or surgical revisions.
Conclusions
The evaluation of this single-center cohort demonstrates that the long-term ITT of chronic pain and severe spasticity is appropriate, safe and efficient. Although, continuous follow-ups are mandatory for warranting a successful management of ITT and troubleshooting in case of adverse events.
References
None
Learning Objectives
1. Long-term intrathecal therapy of chronic pain and severe spasticity is appropriate, safe and efficient.
2. Pump exchanges due to battery depletion should be performed after 6 years.
3. Continuous follow-ups are mandatory for warranting a successful management of ITT and troubleshooting in case of adverse events.
O027 - GOOD PRACTICE GUIDELINES FOR PSYCHOLOGICAL ASSESSMENT AND INTERVENTION FOR NEUROMODULATION SERVICES (ID 187)
Abstract
Introduction
Psychologists in Pain Neuromodulation (PiPiN) is a network of clinical psychologists working in UK neuromodulation services. The group meets regularly to discuss developments in the field of neuromodulation, psychological aspects of surgical care and to share good practice.
A multidisciplinary assessment, including a psychological assessment, is recommended for all patients under consideration for neuromodulation yet there are no existing guidelines to inform these assessments. Furthermore, access to psychological intervention prior to or after surgery can be variable.
This paper makes recommendations for good practice when conducting pre-operative assessments, planning pre or post-surgical psychological intervention and considers some of the professional challenges for psychologists working in neuromodulation services.
Materials / Methods
In light of the lack of standardised criteria for psychologists working in neuromodulation, one of the goals of the guideline development group was to map the arrangements for delivering psychological input within UK neuromodulation services. The mapping exercise was conducted using a short survey circulated to the PIPIN email distribution list.
A systematic review of the literature was conducted to establish the existing evidence regarding psychological predictors of outcome from neuromodulation for pain.
Extensive discussion within the professional network was undertaken to differentiate between factors that were amenable to treatment/intervention and could therefore be considered cautions for neuromodulation, and those that should be exclusions for the same.
Results
Survey data from the professionals invovled in PiPiN showed that, of the 17 centres which responded to the survey, 67% confirmed psychologists were routinely involved in preoperative assessment, whilst 33% had ad-hoc or variable provision. 54% of the centres included psychological input within their prehabilitation programmes, whilst 13% did not offer such a programme. In regard to postoperative care the results were more mixed. 40% of psychologists were routinely involved in following up patients after surgery, 30% offered no routine follow up, 10% made other arrangements and 20% services were unclear whether postoperative support was provided.
The systematic review hightlighted a range of psychological factors that are associated with poorer outcome from neuromodulation. A traffic light system was established that can aid clinical decision making with regards to the psychological appropriateness of neuromodulation. The wider role of psychologists in contributing to the neuromodulation team is also outlined.
Discussion
These guidelines provide a consensus agreement on the role of psychology within neuromodulation services for new and existing services in the UK and beyond.
Conclusions
These guidelines provide a consensus agreement on the role of psychology within neuromodulation services.
References
None
Learning Objectives
1. Outlining the role of psychology within neuromodulation services
2. Providing consenus on the psychological factors that may be identified during preoperative assessment
3. Identifying areas for research and development in the field of psychology and neuromodulation
O031 - ACUTE AND CHRONIC PERFORMANCE, USABILITY, AND SAFETY OF OPTOELECTRONIC RECHARGEABLE NEUROSTIMULATOR FOR VAGUS NERVE STIMULATION (ID 198)
Abstract
Introduction
The innovative technology of this neurostimulator has been developed to meet today's challenges in neuromodulation. First, MRI safety, offering potential for personalized therapy, is achieved through its optoelectronic technology. Optical pulses generated by an implantable pulse generator (IPG) are carried through optical fibers and converted into electrical stimuli at the electrode by ultra-miniature photovoltaic cells. Second, the potential for energy-intensive integrated biomarkers is made possible by its fast rechargeable battery. Third, ease of placement during surgery is provided by an innovative self-sizing spiral cuff electrode design. This study evaluates the performance, usability and safety of this new neurostimulator in sheep.
Materials / Methods
The neurostimulator and the cuff electrode were implanted in a total of thirty healthy sheep. Device usability was evaluated by experienced VNS neurosurgeons during implantation. Device performance was assessed by weekly interrogations of the device (IPG communication and recharge) and recording of the laryngeal motor evocation potential (LMEP) response to VNS through recurrent laryngeal nerve activation. Device safety was assessed by detailed histology reports, as well as daily clinical examination, behavior assessment, blood samples, and weight measurement.
Results
Each step of the implant surgery was rated as "very easy" by experienced VNS neurosurgeons. Mean device implantation time was 13.8 min and electrode cuff placement was 1.5 min. Weekly IPG communication and recharging was successful in all sheep. Full weekly charge time was between 2 and 5 minutes, except for one sheep (5-10 minutes). Usual clinical VNS pulse width parameters were applied, and cough used as a biomarker of the accepted upper stimulation threshold.
Discussion
Nerve stimulation, identified by LMEP recording, was impaired in 2 sheep. For one sheep, it was impossible to record LMEP data or manually feel laryngeal muscle contractions from week 3 to week 14 over the 26 weeks of implantation. In one animal with insufficient stimulation, the electrode was dislodged. Mild swelling of the implantation site without major inflammation was observed in 13/30 sheep. No other stimulation- or device-related adverse events were observed.
Conclusions
This pre-clinical study in 30 sheep has proven the performance, ease of use and safety of this novel VNS neurostimulator. Its innovative will make it possible to respond to the current challenges of neuromodulation by offering MRI safety, energy-intensive embedded biomarkers and greater ease of placement during surgery.
Learning Objectives
* Development of a new biomarker (LMEP) to understand the stimulatiion.
* Creation of a new technology (optoelectronic) to improve the safety in MRI
* Accessibility to a biomarker (fMRI) to understand the stimulationt thanks to the technology optoelectronic of the implant.
O012 - VALIDATION OF APPROPRIATENESS CRITERIA ON PATIENT SELECTION FOR SPINAL CORD STIMULATION IN CHRONIC PAIN: A STUDY PROTOCOL FOR A PROSPECTIVE CLINICAL TRIAL (ID 204)
Abstract
Introduction
In 2019, a European panel developed patient-specific recommendations, considering both clinical and psychosocial factors, on the appropriate referral and selection of patients with chronic pain for spinal cord stimulation (SCS).1 The panel recommendations (not recommended, recommended, strongly recommended) were embedded in an online educational e-health tool, and were studied in a retrospective study, confirming their applicability and validity.2,3 We here present the design of the prospective study and results of a feasibility survey.
Materials / Methods
In this European multicentre study, data from patients who are considered for SCS will be prospectively collected by implant centres that have not previously used the e-health tool for patient selection. Furthermore, the tool should not be used during the conduct of the study. Based on the results from the retrospective study and a feasibility survey with 11 implant centres, the size of the patient population was calculated. At the moment of the final decision on SCS, general baseline data, e-health tool variables and the centre decision on SCS will be documented for all patients, but only those eventually receiving SCS will be followed up long term (figure). Patients not receiving SCS will be excluded because of the likelihood of incomplete data. At the end of the study, the diagnostic value of the panel recommendations for patient outcomes will be determined.
Results
The primary aim of the study is to assess the predictive value of the panel recommendations for patient outcomes. To meet this objective, follow-up data on at least 284 patients receiving SCS needs to be collected. Based on the results of the feasibility survey, on average six patients per month per centre have a positive decision on SCS. Of these patients, approximately 25% will have a negative trial, and therefore 355 patients need to be enrolled to achieve sufficient power. In all but one of the centres participating to the feasibility survey, patient selection is furthermore based on a multidisciplinary assessment, with the majority (9/11) always performing a screening trial.
Discussion
Because of the inclusion of centres without tool experience, an internal pilot study with initially only four centres will be conducted to understand the representation of patient profiles across the panel recommendations.
Conclusions
The prospective validation study aims to determine if patient selection for SCS can be improved by applying the appropriateness criteria embedded in an educational e-health tool. An internal pilot study will be conducted first to resolve uncertainties around the distribution of certain patient profiles.
References
1. Thomson S, Huygen F, Prangnell S, De Andrés J, Baranidharan G, Belaïd H, et al. Appropriate referral and selection of patients with chronic pain for spinal cord stimulation: European consensus recommendations and e-health tool. Eur J Pain 2020;26:1169-81.
2. Thomson S, Huygen F, Prangnell S, Baranidharan G, Belaïd H, Bart Billet, et al. Applicability and Validity of an e-Health Tool for the Appropriate Referral and Selection of Patients With Chronic Pain for Spinal Cord Stimulation: Results From a European Retrospective Study. Neuromodulation 2023;26:164-71.
3. Thomson S, Helsen N, Prangnell S, Paroli M, Baranidharan G, Belaïd H, et al. Patient selection for spinal cord stimulation: The importance of an integrated assessment of clinical and psychosocial factors. Eur J Pain 2022;26:1873-81.
Learning Objectives
1. A retrospective study showed that there is a strong relationship between the panel recommendations embedded in an e-health tool and outcomes after SCS
2. The findings from the retrospective study should be validated prospectively to confirm if the e-health tool can improve patient selection for SCS
3. An e-health tool that can predict treatment outcomes can help physicians and policymakers select the appropriate patients for SCS
O016 - EVIDENCE OF BRAIN STRUCTURAL AND FUNCTIONAL CONNECTIVITY CHANGES AFTER SPINAL CORD TRANSCUTANEOUS STIMULATION-BASED TRAINING FOLLOWING SPINAL CORD INJURY (ID 206)
Abstract
Introduction
Non-invasive spinal cord transcutaneous stimulation (scTS) is a neuromodulatory intervention that has the potential to enhance the therapeutic effects of activity-based training (ABT) on upper extremity (UE) function recovery after a spinal cord injury (SCI). The beneficial effects of scTS on function recovery are thought to be due to spinal and supraspinal plasticity mechanisms. However, brain’s role or modulation in scTS-based rehabilitation remains unclear and under-investigated. This study aims to bridge this gap.
Materials / Methods
We present preliminary results obtained with 5 chronic, cervical, complete and incomplete SCI individuals who participated in an ongoing three-arm, multicenter, open-label randomized controlled trial. The protocol consists of a baseline assessment, 60 sessions of intervention (60 sessions of scTS+ABT, 40 sessions of scTS+ABT followed by 20 sessions of ABT alone, or 20 sessions of ABT alone followed by 40 sessions of scTS+ABT), and re-assessment at post-intervention. During the intervention, each participant received UE ABT, with or without the constant targeted scTS applied depending on participants tolerability and deficits. As part of the outcomes evaluation, SCI participants underwent motor and sensorimotor function testing using the UE motor scale (UEMS) as part of the AIS evaluation and the GRASSP scale, respectively. Grey matter volume and resting-state functional connectivity (FC) were measured using structural and functional magnetic resonance imaging (MRI) before and after the 60 sessions of intervention and were analyzed using Cat12 and Conn v12 respectively, while including Pre-to-Post clinical scores changes as covariates.
Results
All participants tolerated the interventions without serious side-effects. All participants improved their motor and sensorimotor UE function (ΔUEMS = +7.8 ± 5.8; ΔGRASSP = +28.2 ± 11). Considering Pre-to-Post UEMS and GRASSP changes, scTS-based training resulted in an increase of grey matter volume of the right ventral, and dorsal premotor cortex respectively. The protocol also resulted in FC changes between regions of the sensorimotor network and of the prefrontal cortex, positively associated with the increase of the functional UE clinical scores.
Discussion
The results provide evidence of supraspinal effects of scTS-based training in chronic SCI individuals and suggest that scTS is a noninvasive intervention that is also able to modulate cortical networks.
Conclusions
Understanding spinal and supraspinal underlying mechanisms of scTS will help optimize scTS-based rehabilitation after CNS injury.
References
None
Learning Objectives
1. Sixty sessions of scTS-based rehabilitation training improve UE sensorimotor function is individuals with complete and incomplete SCI.
2. scTS-based training induces brain's structural and functional changes involving regions of the frontal and prefrontal cortex.
3. The observed brain changes are associated with the training-induced improved UE function.
O025 - EVOKED COMPOUND ACTION POTENTIAL-CONTROLLED CLOSED-LOOP SPINAL CORD STIMULATION PRODUCES ANALGESIA IN RATS WITH NEUROPATHIC PAIN (ID 210)
Abstract
Introduction
Evoked Compound Action Potentials (ECAPs) represent the neural recruitment of Aβ-fibers in the dorsal column following spinal cord stimulation (SCS)1. In humans, ECAP recordings have been incorporated into a closed-loop SCS (CL-SCS) system that automatically adjusts stimulation intensity to maintain a target ECAP amplitude. Importantly, ECAP-controlled CL-SCS can be superior to open-loop SCS systems in humans2,3. We recently showed that ECAPs can be reliably recorded from naive rats4. Here, we demonstrate for the first time the application of ECAP-controlled CL-SCS in neuropathic rats and its efficacy for pain relief.
Materials / Methods
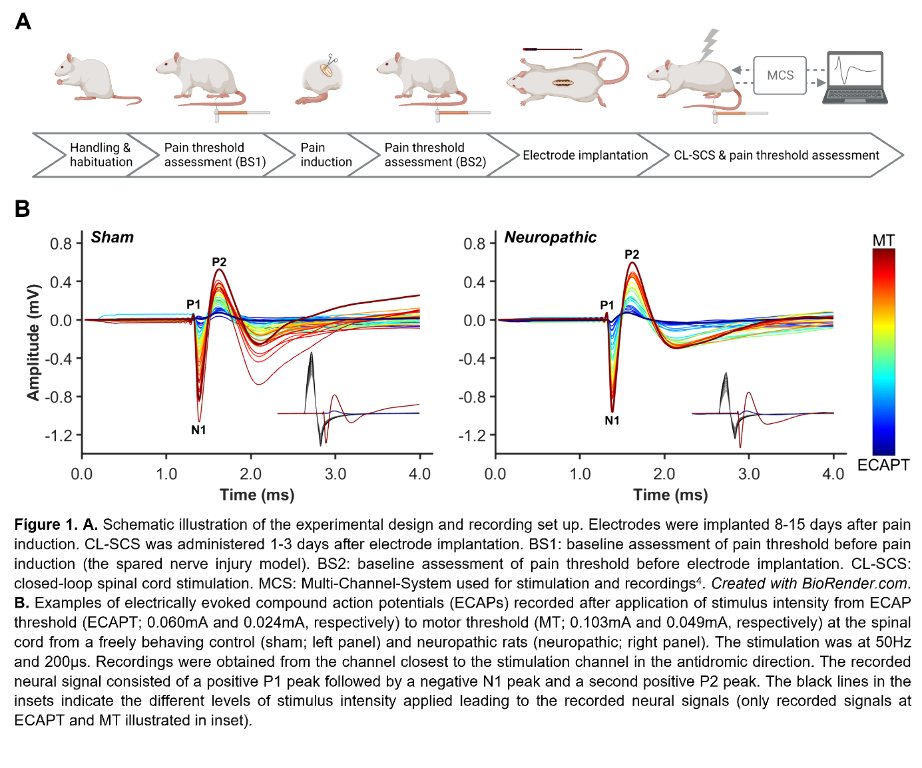
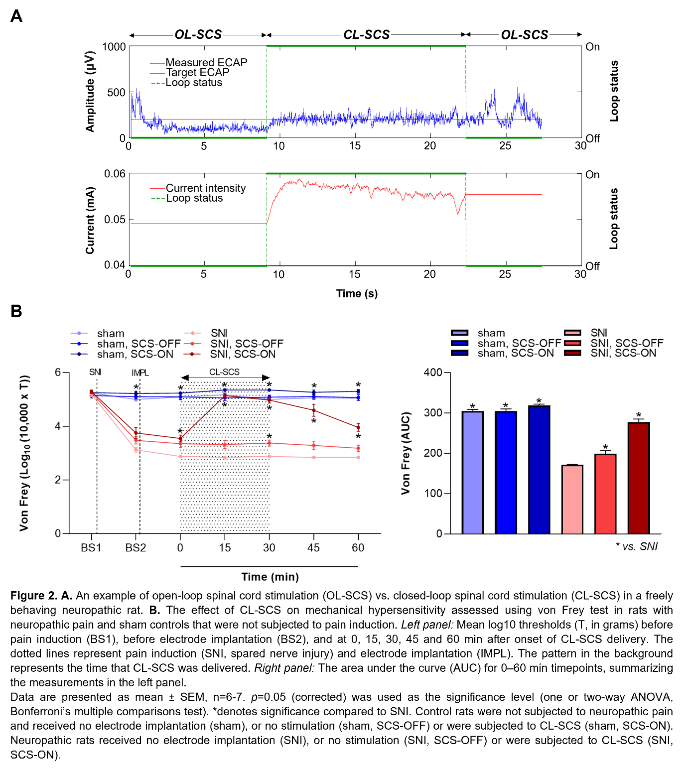
Figure 1A illustrates the experimental steps. Adult male Sprague–Dawley rats (200-300g) were subjected to neuropathic pain using the spared nerve injury model. A custom-made six-contact electrode4 was implanted epidurally covering T11-L3, as confirmed by CT or X-ray. Stimulation and recording were performed in freely behaving animals as previously described4. One contact was used for stimulation and recordings were made from the remaining contacts. The CL-SCS was delivered at 50Hz and 200µs for 30 minutes. To determine analgesic effects from stimulation, paw withdrawal thresholds to mechanical stimuli were assessed using the von Frey test. Protocols were approved by the UK Home Office.
Results
Figure 1B shows ECAPs recorded from neuropathic and sham (control) rats using 50Hz and 200µs stimulation. As expected, the recorded ECAPs showed a characteristic triphasic morphology and the amplitude (P2-N1, mV) increased as higher currents (mA) were applied. Figure 2A illustrates that these recordings allowed for successful application of ECAP-controlled CL-SCS in freely behaving neuropathic and sham rats. The analgesic effects of CL-SCS are shown in Figure 2B. It provided a significant reduction of mechanical hypersensitivity assessed by the von Frey test. The strongest analgesic effect was observed during the application of CL-SCS. However, after the termination of CL-SCS the effect gradually declined up to 30 minutes after the application of CL-SCS. CL-SCS had no effect in sham rats not subjected to neuropathic pain.
Discussion
This is the first demonstration of analgesic effects resulting from the application of ECAP-controlled CL-SCS in neuropathic rats.
Conclusions
This novel observation, together with our published work on the reliability of ECAP recordings in rats4, allow better translation of pre-clinical SCS models as our method is more closely aligned to human stimulation protocols. Our results also elucidate the mechanisms of SCS action to further improve future clinical SCS applications.
References
(1) Parker, J., Karantonis, D. & Single, P. Hypothesis for the mechanism of action of ECAP-controlled closed-loop systems for spinal cord stimulation. Healthc Technol Lett 7, 76–80 (2020).
(2) Mekhail, N. et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol 19, 123–134 (2020).
(3) Mekhail, N. et al. Durability of Clinical and Quality-of-Life Outcomes of Closed-Loop Spinal Cord Stimulation for Chronic Back and Leg Pain: A Secondary Analysis of the Evoke Randomized Clinical Trial. JAMA Neurol 79, 251–260 (2022).
(4) Dietz, B. E., Mugan, D., Vuong, Q. C. & Obara, I. Electrically Evoked Compound Action Potentials in Spinal Cord Stimulation: Implications for Preclinical Research Models. Neuromodulation 25, 64–74 (2022).
Learning Objectives
(1) Describe the novel approach allowing reliable recording of ECAPs from the dorsal column axons in freely behaving neuropathic and control rats.
(2) Interpret experimental outcomes showing that ECAP-controlled CL-SCS produces analgesia in neuropathic rats.
(3) Explain how the use of ECAP-controlled CL-SCS in animal models may enable better translations between pre-clinical and clinical research in SCS.
