Chiara Fabris, United States of America
University of Virginia Center for Diabetes TechnologyPresenter of 3 Presentations
REDUCTION OF POSTPRANDIAL HYPOGLYCEMIA IN INDIVIDUALS WITH TYPE 1 DIABETES USING AN INSULIN SENSITIVITY-INFORMED BOLUS CALCULATOR AFTER AN AEROBIC EXERCISE SESSION
- Chiara Fabris, United States of America
- Ralf Nass, United States of America
- Jennifer Pinnata, United States of America
- Chase Phillips, United States of America
- Christian Wakeman, United States of America
- Mary Clancy-oliveri, United States of America
- Daniel Chernavvsky, United States of America
- Marc Breton, United States of America
Abstract
Background and Aims
Insulin sensitivity (SI) fluctuations, e.g. driven by physical activity and exercise, complicate insulin dosing and worsen glycemic control in individuals with type 1 diabetes (T1D). At last-year ATTD, we presented in-silico results demonstrating the benefit of using an SI-informed bolus calculator to account for exercise-induced SI changes. This year, we present the results from the first clinical deployment of the SI-informed system.
Methods
Fifteen subjects with T1D (male/female:10/5, age:45.1±12.6years, HbA1c:6.9±0.9%) using continuous glucose monitor (CGM) and insulin pump completed a 4-week at-home data collection, followed by two 24-hour hotel admissions. During the admissions, participants engaged into a 45-minute early-afternoon aerobic exercise session, after which they received a standardized dinner meal. The dinner bolus was computed using a standard or SI-informed bolus calculator; the latter modulates the insulin dose according to the deviation between usual SI estimated from historical data and real-time SI. Postprandial glycemic control was assessed by CGM-based low/high blood glucose indices (LBGI/HBGI) and percent time outside 70-180mg/dL, and compared between the two admissions.
Results
A 31% exercise-induced SI increase was observed (p=0.0002). The corresponding bolus modulation allowed to reduce postprandial hypoglycemia (ΔLBGI=(–)1.3, p=0.006; ΔPERC<70=(–)6.7%, p=0.049), without significant increase in hyperglycemia (ΔHBGI=1, p=0.075; ΔPERC>180=2.8%, p=0.5) [see figure]; the total number of administered hypoglycemia treatments was reduced from 12 to seven. Sensor glucose at dinnertime did not differ between the admissions (ΔCGM,DINN=(–)0.7mg/dL, p=0.925).
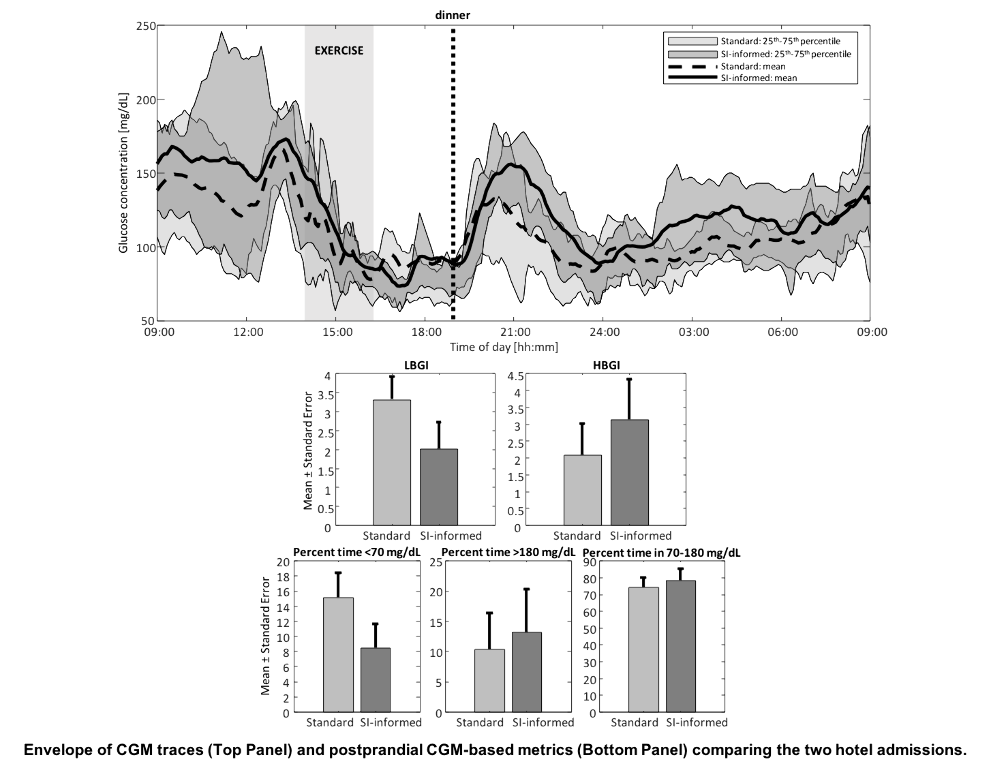
Conclusions
This pilot study shows the safety and efficacy of using the proposed SI-informed bolus calculator in individuals with T1D. Future studies will be devoted to further testing the method.
Biosensing, diabetes data science and decision support
Abstract
Background and Aims
Real-time data sources are abundant and doubling every two years, with many based on biosensors and relevant to healthcare. This trend is particularly prominent in diabetes, where continuous glucose monitoring (CGM) revolutionized the assessment of key clinical parameters and opened a new research and training field–Diabetes Data Science.
Methods
The high temporal density of CGM data requires new metrics and analytics that take advantage of contemporary methods, including artificial intelligence, machine learning, and data farming. To be relevant, any contemporary CGM-based decision support system (DSS) needs to utilize these types of approaches.
Results
This presentation continues the review of methods enabling automated DSS that we initiated a year ago, and discusses updates, such as the use of a stochastic-process approximation of the day-to-day variation in human physiology and behavior to individualize and optimize diabetes control. Pattern recognition, clustering, and classification are at the core of this approach, in which the collection of all possible daily patterns, is defined by machine-learning classification of CGM profiles into clusters derived from a library of over 50,000 CGM daily profiles accumulated by recent clinical trials.
Conclusions
CGM daily profiles can be classified into identifiable clusters and within each cluster the individual CGM profiles are similar. Over time, each person transitions through a sequence of clusters, which contribute differently to glycemic control. Treatment adaptation is assisted by presenting pattern/risk predictions, with the goal to maximize the probabilities for favorable daily transitions.
ESTIMATION OF HEMOGLOBIN A1C FROM CONTINUOUS GLUCOSE MONITORING DATA IN INDIVIDUALS WITH TYPE 1 DIABETES: IS TIME IN RANGE ALL WE NEED?
Abstract
Background and Aims
Glycated hemoglobin (A1c) has long been considered the gold-standard metric for quality of glycemic control in diabetes and key predictor of long-term complications. At last-year ATTD, an international panel of experts convened to provide guidance on interpreting and reporting continuous glucose monitoring (CGM) data, recognized time in target range (TIR) as an important metric to complement A1c. In this work, we bridge the gap between the two metrics and present a method to derive estimates of A1c from TIR.
Methods
Data from the International Diabetes Closed-Loop Trial included CGM measurements from N=168 adults with type 1 diabetes over six months, and A1c samples at three and six months. Hemoglobin glycation and clearance were modeled through a first-order differential equation driven by a linear function of daily TIR. A population model was trained on half of the subjects and tested without modifications on the remaining half. The model was then personalized by identifying an individual glycation-rate coefficient using the first A1c sample. Measured and estimated A1c at six months were compared in terms of mean absolute difference (MAD), mean absolute relative difference (MARD), and correlation coefficient (R); 100 replicates of training/testing were performed to assess robustness.
Results
Compared to the population-based model, personalization with individual glycation rates decreased MAD (0.43% to 0.25%) and MARD (6.1% to 3.56%), and increased R (0.77 to 0.93 [figure]).
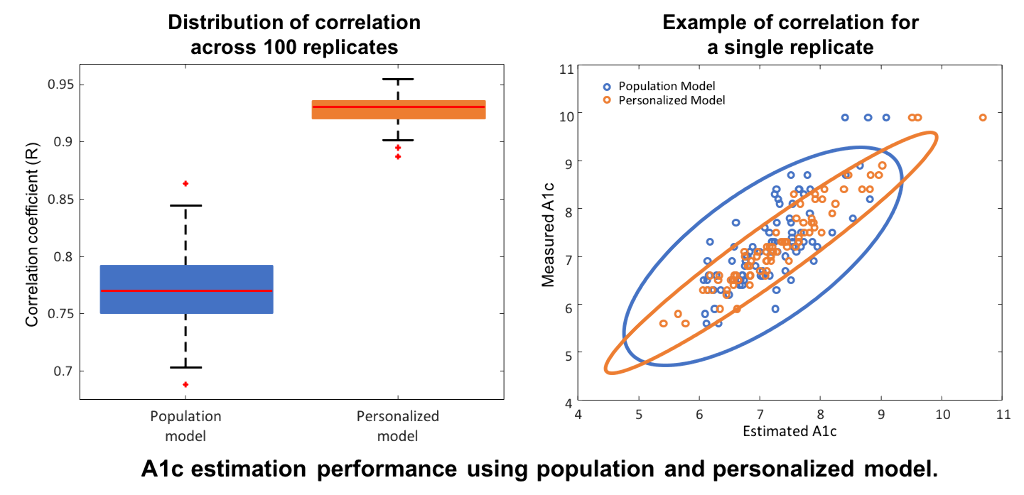
Conclusions
The TIR-A1c relationship is mediated by identifiable individual glycation coefficients. Following personalization, TIR provides high-precision A1c estimates, therefore representing a viable alternative to A1c in research and clinical-practice settings.
