
Presenter of 1 Presentation
ASSOCIATIONS BETWEEN IRON DEPOSITION IN THE BRAIN AND AD-LIKE NEUROIMAGING PHENOTYPES IN COGNITIVELY UNIMPAIRED ADULTS
Abstract
Aims
In cognitively unimpaired (CU) individuals, we aimed to explore the impact of cerebral iron deposition on the brain structural properties in selected regions that are vulnerable to AD pathology.
Methods
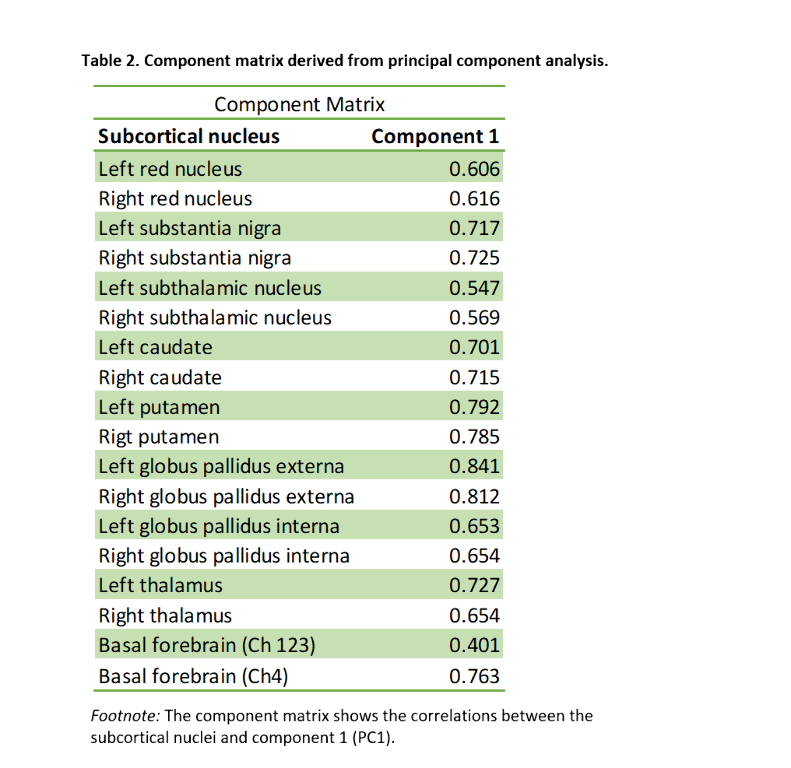
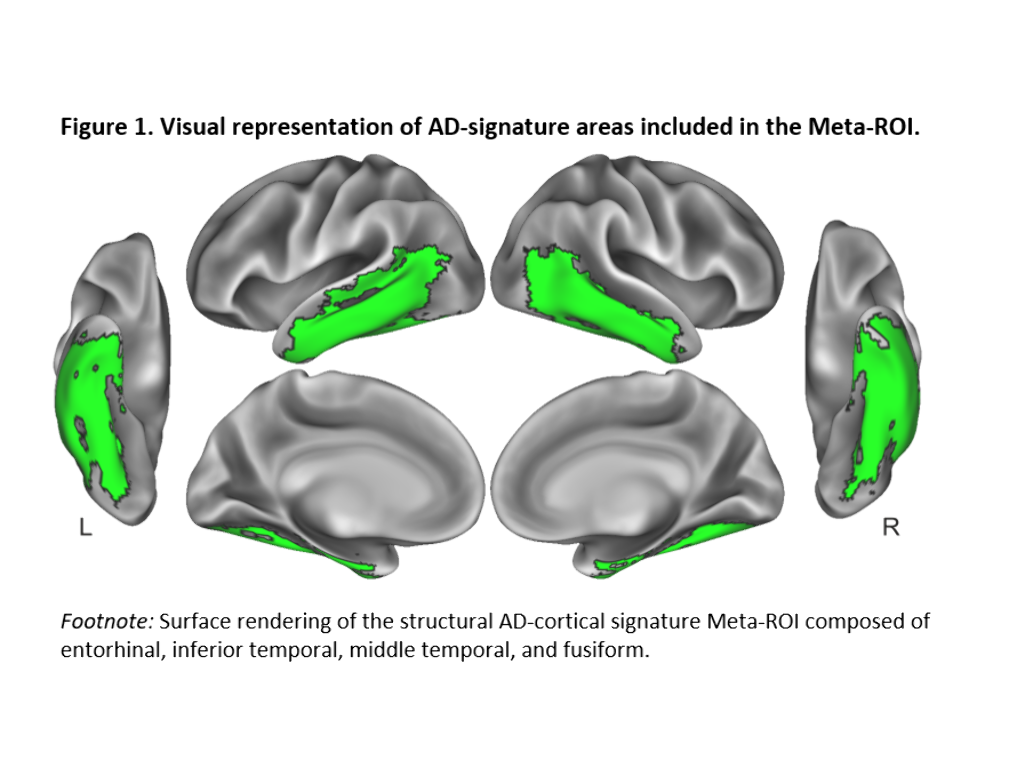
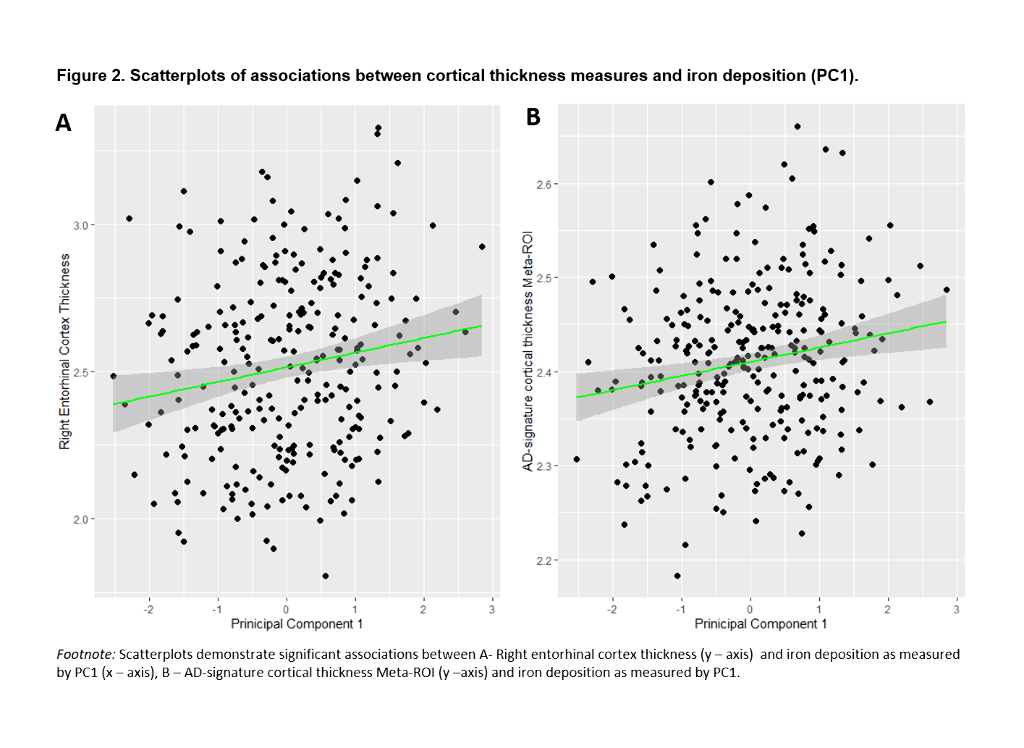
We included 288 CU adults from the ALFA+ cohort (Table 1). T2-weighted images were used to calculate mean hypointensity values in 18 subcortical nuclei. Due to the high correlations among regional hypointensities, we performed a principal component analysis (PCA) to reduce dimensionality (Table 2). T1-weighted images were used to measure cortical thickness (CT) using FreeSurfer v6.0, from which AD cortical signature (Meta-ROI) and the entorhinal cortex (ERC) values were then derived (Figure 1). We created separate general linear models where regional CT was the dependent variable, while the first principal component (PC1), age, sex, APOE-ε4 status, and education level were entered as predictors. In additional models, CSF levels of amyloid-beta (Aβ) ratio (Aβ42/40) and phosphorylated tau (p-tau) were entered as covariates. Statistical significance was set at p<0.05 uncorrected for multiple comparisons.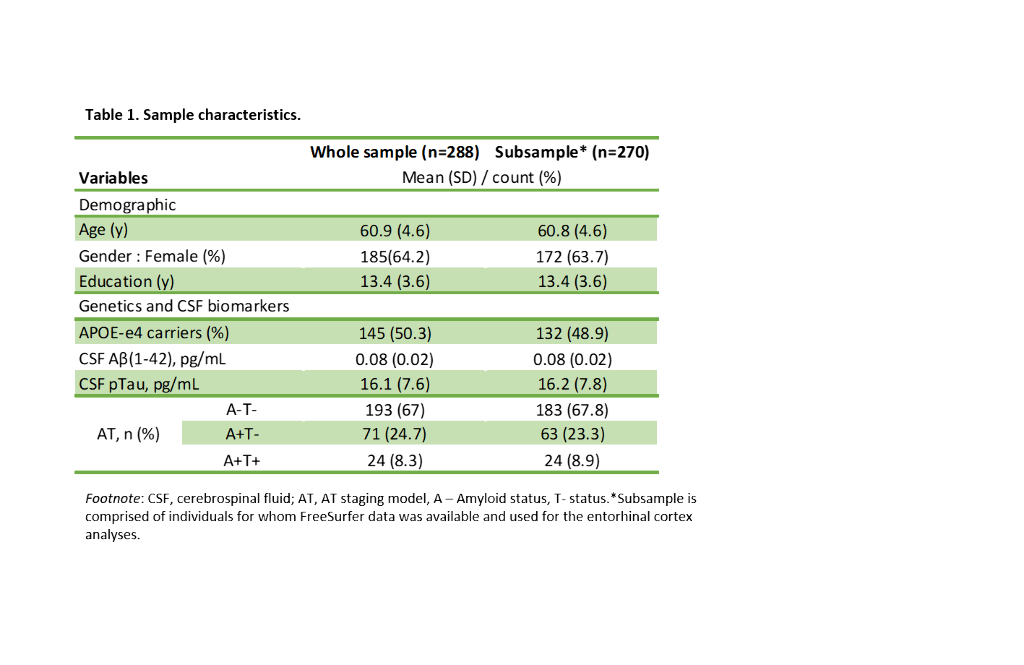
Results
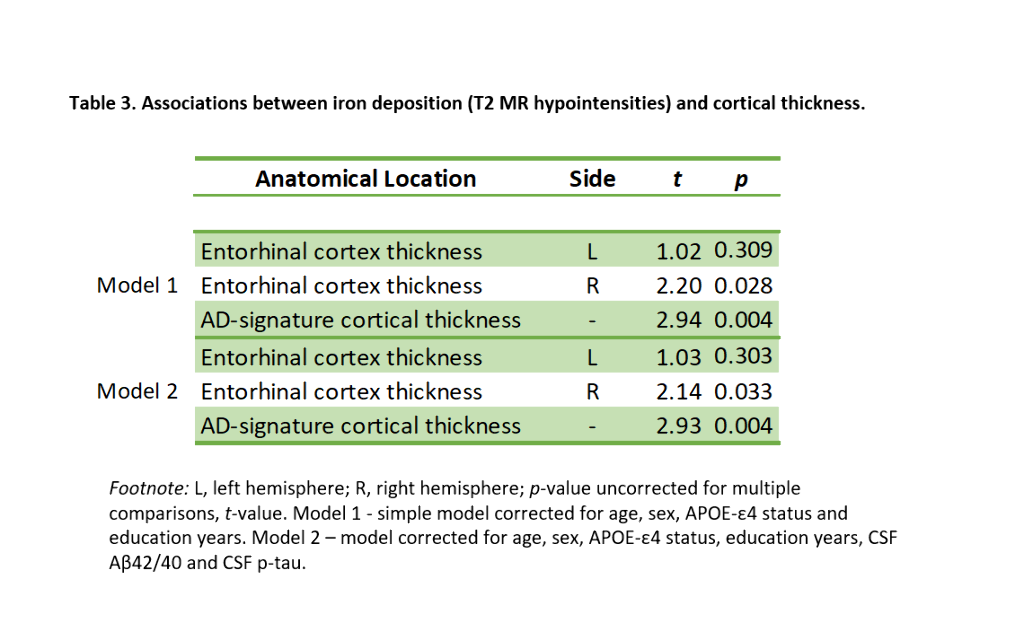
PC1 explained 47.64% of the variance of intensities in subcortical regions. Lower PC1 values, denoting, higher iron concentration, were significantly associated with lower right ERC and AD-signature thickness (Table 3, Figure 2). These results remained significant once CSF core AD biomarkers were entered into the model.
Conclusions
In CU individuals, iron deposition is associated with AD-like neuroimaging pattern independently of AD biomarkers. These results suggest that iron accumulation might be associated with structural brain changes that render the brain more vulnerable to the effects of AD pathology.
