Welcome to the AD/PD™ 2022 Interactive Program
The conference will officially run on Central European Time (CET) - Barcelona Time
To convert the conference times to your local time Click Here
DATA-DRIVEN SUBTYPING AND POST HOC ANALYSIS OF AZD0530 (FYN) TRIAL
Abstract
Aims
The high failure rate of clinical trials in AD, coupled with increasing evidence of phenotypic and temporal heterogeneity in observational cohorts, suggests that traditional inclusion criteria may be inadequate. Computational disease progression models can unravel this heterogeneity to potentially select a homogeneous cohort with increased sensitivity to treatment effect. This study investigated whether such a model can identify and characterize heterogeneity within the phase 2a “FYN” clinical trial (NCT02167256).
Methods
Subtype and Stage Inference (SuStaIn) was used to learn subtypes of cumulative abnormality in regional brain volumes. After training on ADNI data (N=443), the model was deployed to assign subtype and stage to FYN data (N=159) at screening. A post hoc subgroup analysis was performed in these subgroups following the trial protocol. The primary outcome was brain glucose uptake (18F-FDG PET) over 52 weeks, with MRI and neuropsychological tests as secondary outcomes.
Results
SuStaIn identified a subcortical (S1) and cortical (S2) subtype in ADNI (Figure 1), distinguished by regions involved earlier in each progression. The cortical subtype exhibited greater cognitive impairment (t-test, ADAS-13, p<0.001). The model revealed similar heterogeneity in FYN (Figure 2) and the cortical subtype (S2) showed potential treatment benefit in both secondary outcomes: ADAS-Cog-12 (Figure 3) and vMRI measures (Figure 4), but not in the primary outcome (Figure 5).
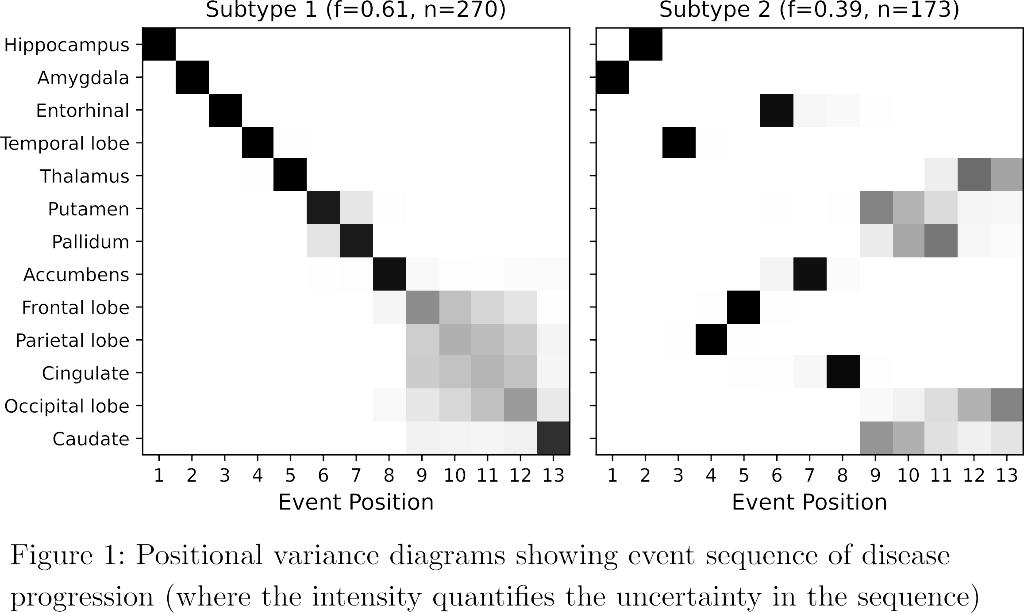
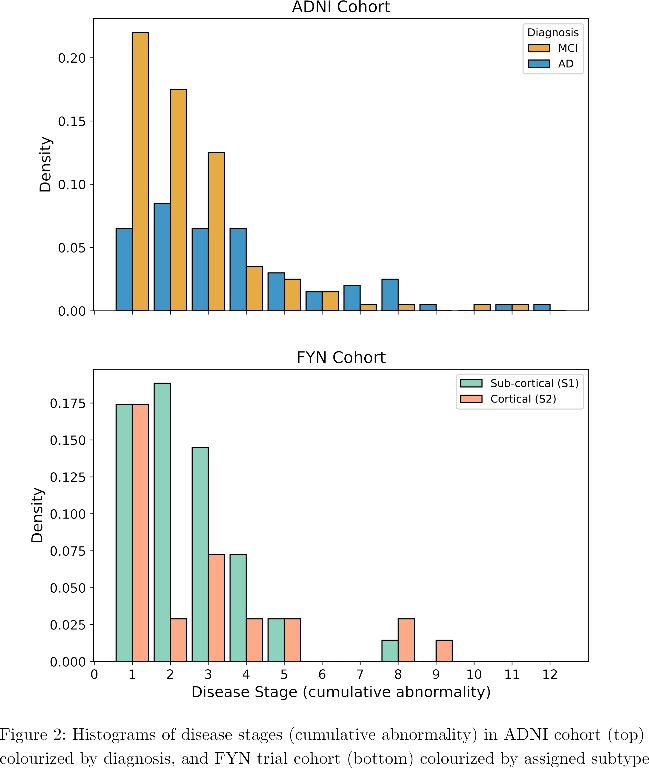
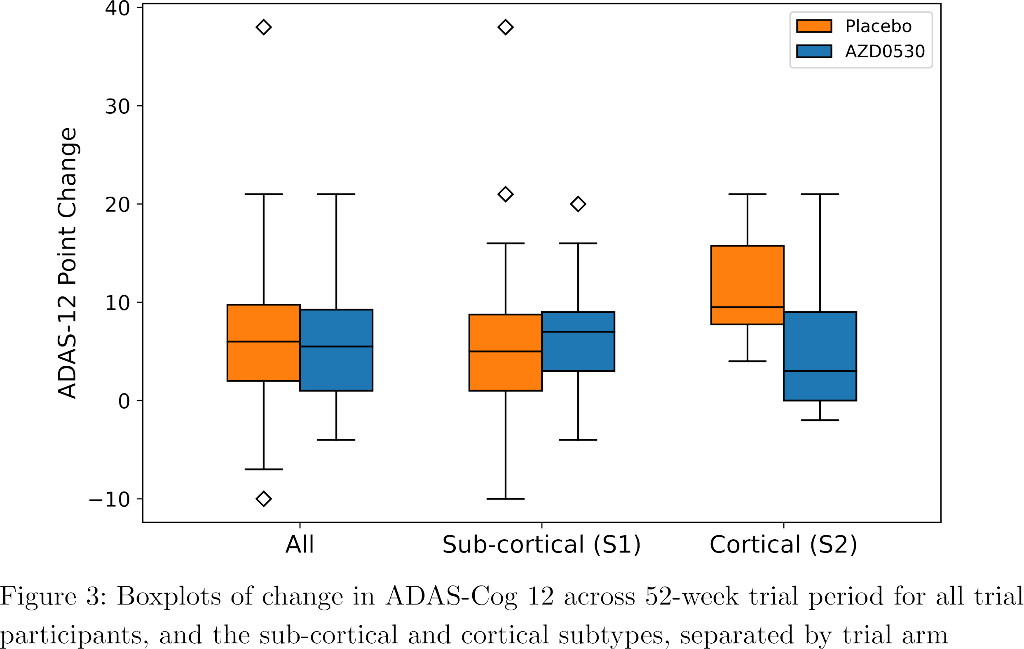
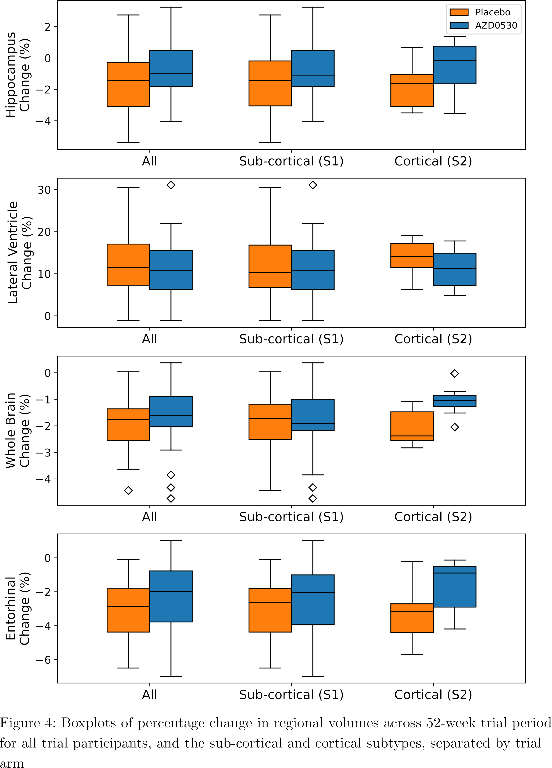
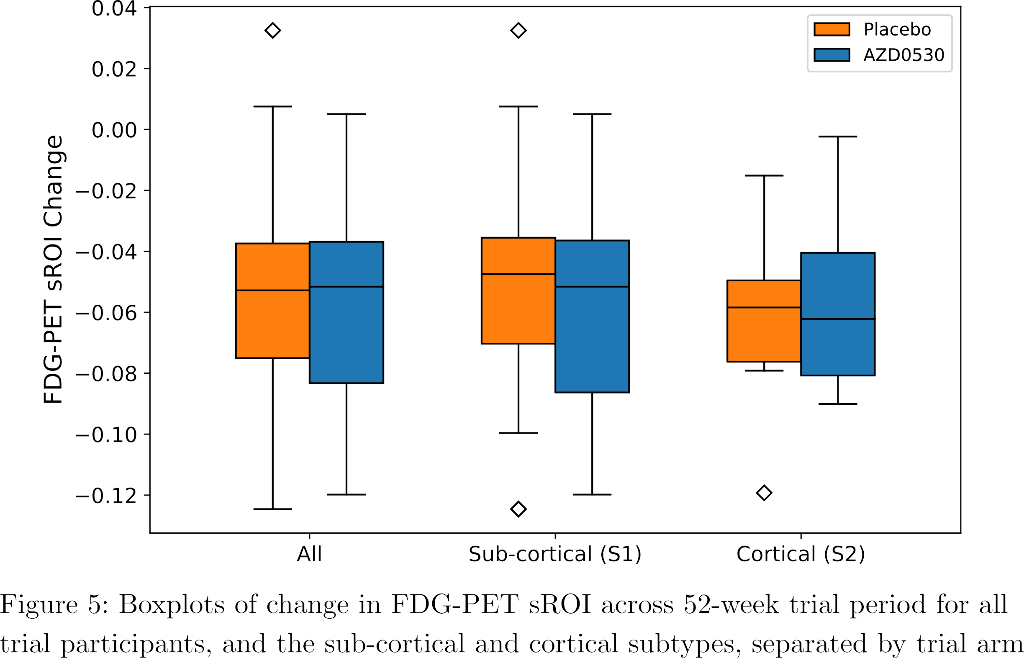
Conclusions
Our model characterized phenotypic and temporal heterogeneity in the AZD0530 trial cohort, and identified potential treatment effect in a post hoc subgroup, although the small sample size compels further investigation such as in Phase 3 trials.
ACTIVE DEEP LEARNING TO DETECT COGNITIVE IMPAIRMENT IN ELECTRONIC HEALTH RECORDS
Abstract
Aims
Dementia is under-recognized in the community, under-diagnosed by healthcare professionals, and under-coded in claims data. Timely diagnosis of dementia, however, is important for patients and their caregivers to plan for the future. We aim to screen electronic health records (EHR) for signs of cognitive impairment.
Methods
To identify patients with cognitive impairment in EHR, we applied a deep learning based natural language processing (NLP) algorithm to unstructured clinician notes and compared the model’s performance to a baseline model that used regularized logistic regression with structured data (dementia related diagnosis codes and medication). We trained and evaluated the algorithm with a seed set of patients with detailed chart review and adjudication of cognitive status by a team of experts. Next, we used an active learning loop to continually improve model performance and select candidate cases for labeling using a combination of uncertainty and diversity measures.
Results
The baseline model with diagnosis codes and medications trained on the initial seed set (N=943) had an area under the receiver operating characteristic curve (AUROC) of 0.79. The deep learning model improved the AUROC to 0.92 and increased sensitivity of dementia detection from 0.59 to 0.79, at similar level of specificity. Of the patients which were predicted positive, 16.1% did not have a dementia-related diagnosis code suggesting that many patients with cognitive impairment may be undiagnosed.
Conclusions
Automatic processing of EHR with deep learning tools can be used to screen for patients with cognitive impairment who could benefit from a cognitive evaluation or be referred to specialist care.
COGNITIVE SCREENING IN PATIENTS WITH PARKINSONISM: PROPOSAL FOR A NEW, MACHINE LEARNING-BASED DIAGNOSTIC TOOL
Abstract
Aims
The assessment of cognitive deficits is crucial in the field of parkinsonisms diagnosis and management. To evaluate cognitive profile, screening tests are generally preferred, since in-depth neuropsychological batteries are reliable but time-consuming. Otherwise, low-level of correspondence is observed, when the two evaluations are compared.
A new tool, we named CoMDA (Cognition in Movement Disorders Assessment), was composed by merging all items, without repetition, of Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA) and Frontal Assessment Battery (FAB). Moreover, a machine learning was developed to classify the CoMDA score and to reach accurate prediction of risk of dementia.
Methods
500 patients (400 with Parkinson’s disease, 41 with vascular parkinsonism, 31 with progressive supranuclear palsy, 28 with multisystem atrophy) underwent CoMDA (Level 1) and in-depth neuropsychological battery (Level 2), considered as the gold standard for classification of cognition
Results
Referring on Level 2, the rate of false negatives was 55,7% for MMSE, 45,6% for FAB and 57,4% for MoCA. Otherwise, the classification obtained with CoMDA dropped to 31,7% the false negative rate. Considering Level 2 as a 3-level continuous feature, a Machine Learning model was developed to classify L2 feature accurately and with adequate cross-generalization capacity. CoMDA-ML produce the accurate and generalizable L2 predictions (micro average ROC curve, AUC = .81; and AUC=.85, .67 and .83 for L2 individual classes respectively).
Conclusions
CoMDA and COMDA-ML are reliable and time-sparing instruments, accurate in distinguishing cognitively impaired patients from those with normal cognition.
This study has been registered on ClinicalTrials.gov (NCT04858893).
COMPARISON OF STRUCTURAL AND METABOLIC BIOMARKERS FOR BRAIN AGE PREDICTION USING MACHINE LEARNING
Abstract
Aims
Brain age (BA) is commonly assessed by predicting chronological age (CA) from neuroimaging data of healthy individuals by means of machine learning. During aging, the adult brain undergoes changes both on the morphological and metabolic level. To date, however, BA remains almost exclusively predicted from structural MRI scans. Here, we compare structural (MRI) and metabolic (18F-FDG-PET) biomarkers of neurodegeneration as potential predictors of BA.
Methods
Matched MRI and 18F-FDG-PET scans of 362 cognitively unimpaired individuals were acquired from the ADNI database (adni.loni.usc.edu). Mean gray matter volume and standardized uptake value ratios were calculated from 216 regions of spatially normalized MRI and 18F-FDG-PET scans, respectively. Regression models were trained to predict BA from MRI or 18F-FDG-PET scans using 70% of the data, while the remaining 30% (n = 110) were used for performance evaluation. Mean absolute error (MAE) and R² were assessed between BA and CA, and subsequently compared across the two neuroimaging modalities. Finally, correlations between MRI- and 18F-FDG-PET-predicted BA and neuropsychological test scores from the same visit were calculated.
Results
BA predicted from MRI- and 18F-FDG-PET showed an MAE (R²) of 3.8 (0.5) and 3.71 years (0.53), respectively, and was weakly correlated between modalities. Higher 18F-FDG-PET-, but not MRI-predicted BA, was associated with worse cognitive function.
Conclusions
We deliver first insights that 18F-FDG-PET is useful in the assessment of BA. The established metabolic BA appears to be more sensitive to differences in cognitive function in cognitively unimpaired individuals compared to structural BA.
EEG ABNORMALITIES IN PATIENTS WITH DELIRIUM AS A PREDICTIVE BIOMARKER OF DEMENTIA WITH LEWY BODIES
Abstract
Aims
Delirium is an acute neurological dysfunction, characterized by altered conscious state, akin to fluctuating cognition. It is frequent in elderly patients when hospitalized and is characterized by an increased risk of developing dementia, especially Dementia with Lewy Bodies (DLB). EEG Prominent posterior slow-wave activity in the pre-alpha/ theta range is a supportive biomarker for DLB diagnosis from the prodromal stage. This EEG pattern correlates with fluctuating cognition and is the electrophysiological underpinning of the so called thalamo-cortical dysrhythmia. We aim to assess whether non demented hospitalized patients with delirium show specific QEEG abnormalities typical of thalamo-cortical dysrhythmia.
Methods
24 consecutive patients admitted to Neurology Clinic of University of Chieti were administered Mini Mental State Examination (MMSE) as per clinical routine. The presence of delirium was assessed by 4AT test. All subjects underwent resting state EEG recording.
Results
11 (7 females) presented delirium (delirium, D) according to 4AT (9 with hypokinetic, 2 with hyperkinetic type), 13 (5 females) did not show it (controls, C). The two groups did not differ for age and gender. In all but one D (91%), we observed alterations of EEG parameters (DF lower than alpha with increased DFV (>1.2 Hz)). In C, DF and DFV were normal in 92% , (alpha DF and DFV<1.2 Hz) (DF in D vs. C p=0.002 ).
Conclusions
We found strong correlation between the presence of delirium and EEG abnormalities typical of thalamocortical dysrhythmia and of DLB. Our evidence suggests a pathophysiological explanation for delirium. EEG may represent a powerful tool to detect delirium.
VISIT-TO-VISIT BLOOD PRESSURE VARIABILITY PREDICTS CEREBROSPINAL FLUID ALZHEIMER’S DISEASE BIOMARKERS IN OLDER ADULTS
Abstract
Aims
1. Objectives: Blood pressure variability is an emerging risk factor for dementia, but mechanisms remain unclear. We examined whether visit-to-visit blood pressure variability is related to cerebrospinal fluid Alzheimer's disease biomarker levels over time, and whether associations differed by apolipoprotein ε4 carrier status.
Methods
2. Methods: 466 Alzheimer's Disease Neuroimaging Initiative participants without dementia underwent 3-4 blood pressure measurments over 12 months and >= 1 collection of cerebrospinal fluid amyloid-beta and phosphorylated tau samples thereafter. Only biomarker samples collected after the final blood pressure measurement were analyzed. Bayesian linear growth modeling investigated the role of blood pressure variability, apolipoprotein ϵ4, and the passage of time on biomarker levels after controlling for several variables, including average blood pressure and baseline hypertension.
Results
3. Results: Elevated blood pressure variability was associated with increased phosphorylated tau (ß: 3.75 [95% CI 2.12, 5.32]) and decreased amyloid-beta cerebrospinal fluid levels (ß: -2.86 [95% CI -6.05, -.33]) at follow-up. Apolipoprotein ϵ4 carriers with elevated blood pressure variability had the fastest increase in phosphorylated tau levels (ß: 19.34 [95% CI 7.80, 30.62]). Blood pressure variability was not significantly related to amyloid-beta levels over time based on ϵ4 carrier status.
Conclusions
4. Conclusions: Older adults with elevated blood pressure variability exhibit increased cerebrospinal fluid phosphorylated tau and decreased amyloid-beta over time, suggesting blood pressure variability may be a useful marker of Alzheimer’s disease progression. Apolipoprotein ϵ4 carrier status moderated the relationship between blood pressure variability and phosphorylated tau but not amyloid-beta, consistent with other studies linking hemodynamic factors to tau changes.
ASSOCIATIONS BETWEEN IRON DEPOSITION IN THE BRAIN AND AD-LIKE NEUROIMAGING PHENOTYPES IN COGNITIVELY UNIMPAIRED ADULTS
Abstract
Aims
In cognitively unimpaired (CU) individuals, we aimed to explore the impact of cerebral iron deposition on the brain structural properties in selected regions that are vulnerable to AD pathology.
Methods
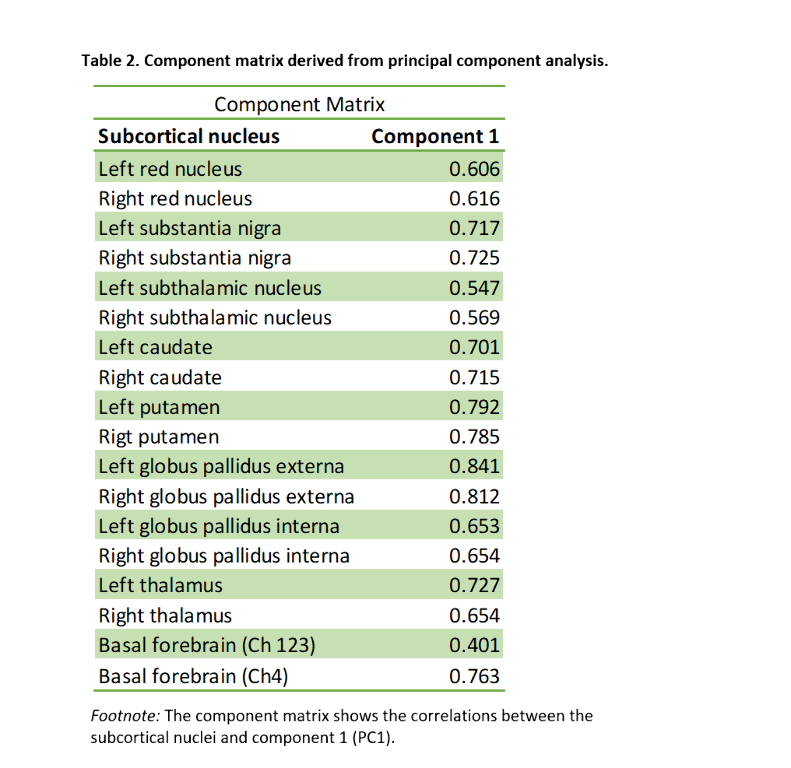
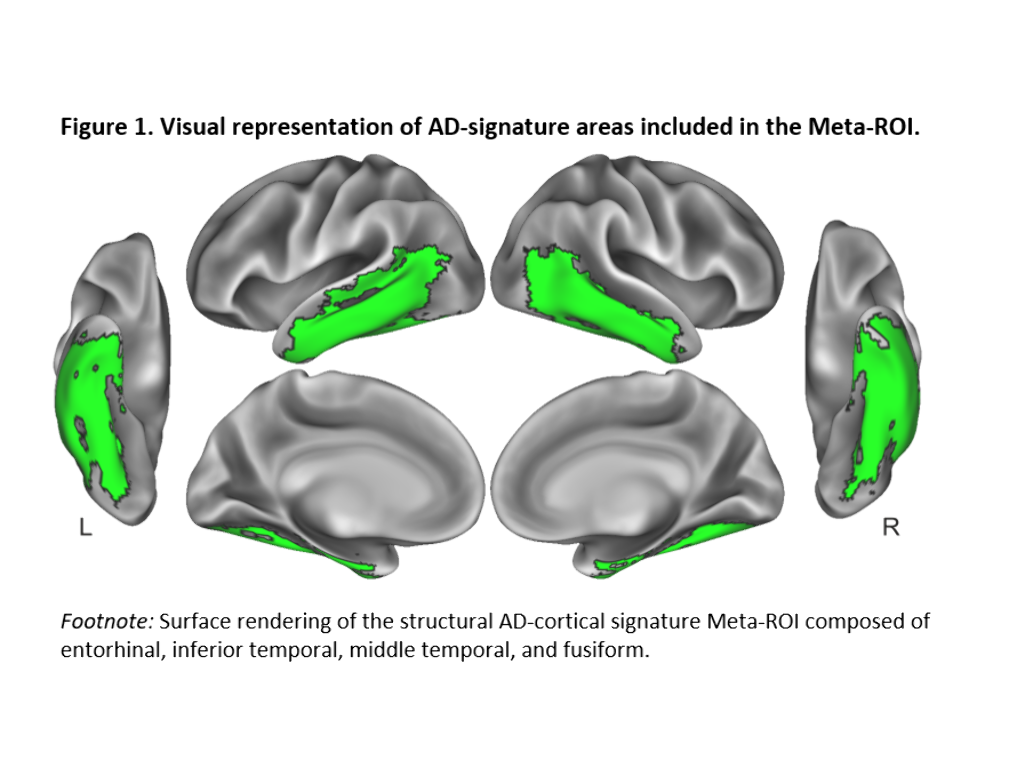
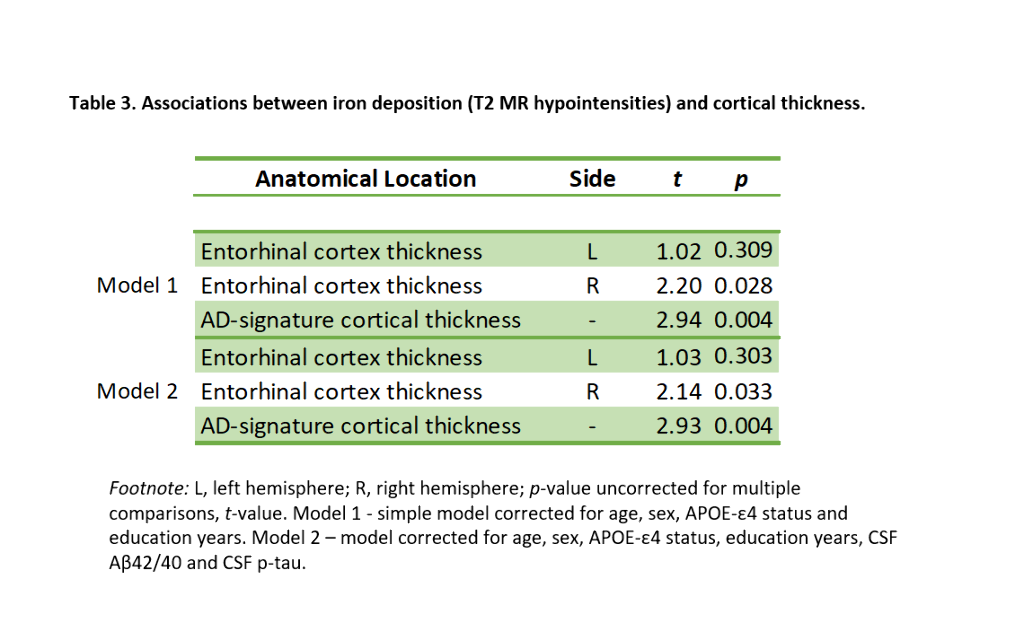
We included 288 CU adults from the ALFA+ cohort (Table 1). T2-weighted images were used to calculate mean hypointensity values in 18 subcortical nuclei. Due to the high correlations among regional hypointensities, we performed a principal component analysis (PCA) to reduce dimensionality (Table 2). T1-weighted images were used to measure cortical thickness (CT) using FreeSurfer v6.0, from which AD cortical signature (Meta-ROI) and the entorhinal cortex (ERC) values were then derived (Figure 1). We created separate general linear models where regional CT was the dependent variable, while the first principal component (PC1), age, sex, APOE-ε4 status, and education level were entered as predictors. In additional models, CSF levels of amyloid-beta (Aβ) ratio (Aβ42/40) and phosphorylated tau (p-tau) were entered as covariates. Statistical significance was set at p<0.05 uncorrected for multiple comparisons.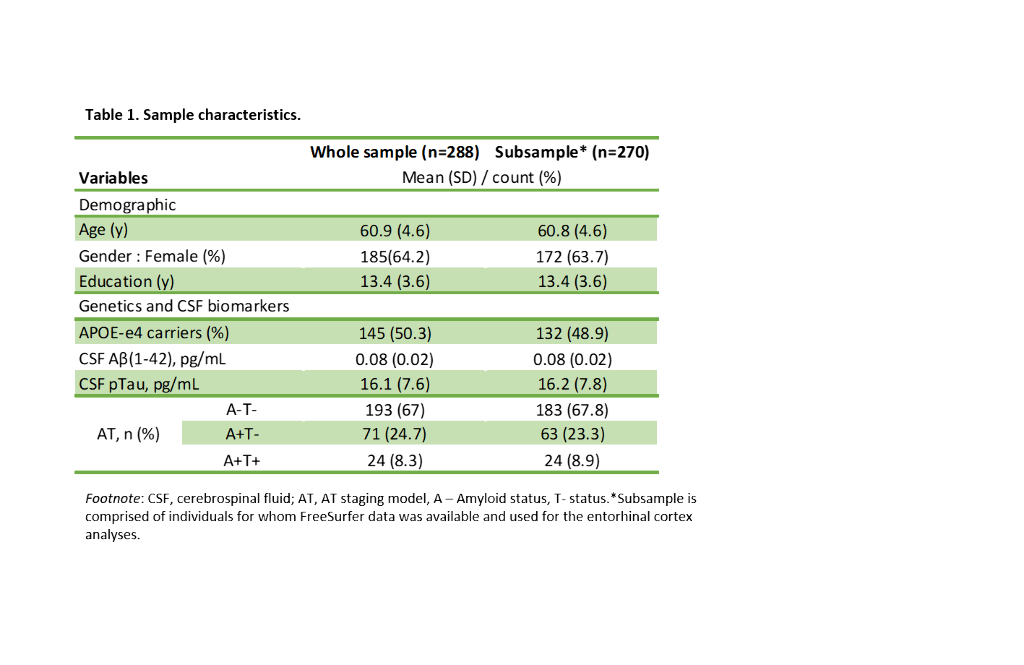
Results
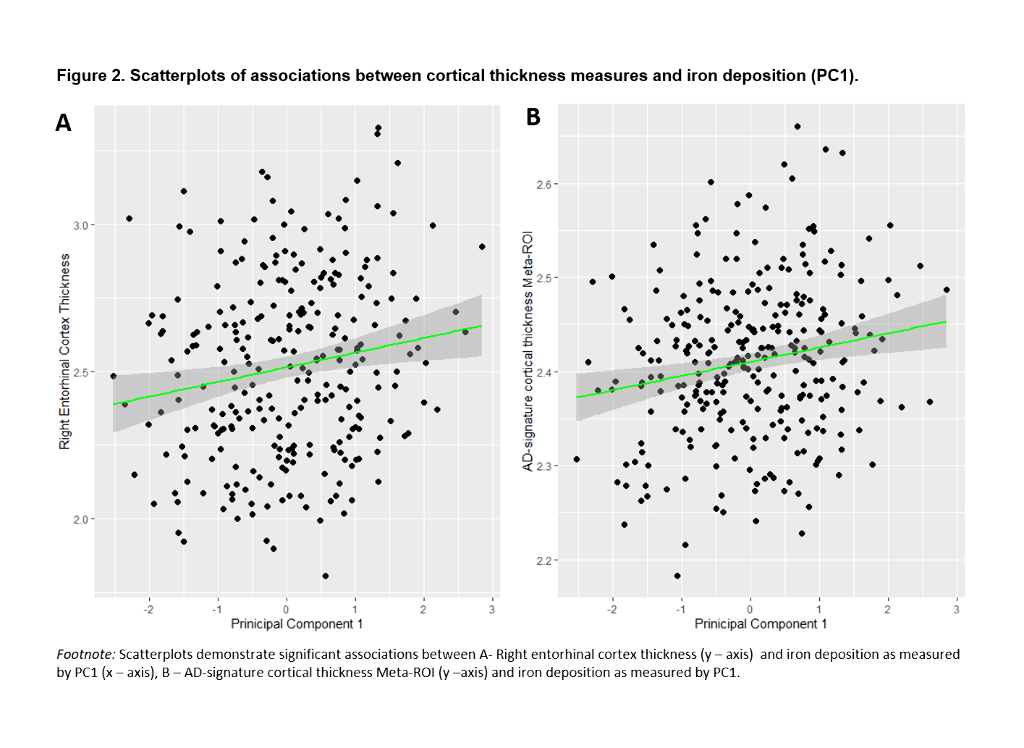
PC1 explained 47.64% of the variance of intensities in subcortical regions. Lower PC1 values, denoting, higher iron concentration, were significantly associated with lower right ERC and AD-signature thickness (Table 3, Figure 2). These results remained significant once CSF core AD biomarkers were entered into the model.
Conclusions
In CU individuals, iron deposition is associated with AD-like neuroimaging pattern independently of AD biomarkers. These results suggest that iron accumulation might be associated with structural brain changes that render the brain more vulnerable to the effects of AD pathology.
QUANTITATIVE DISSECTION OF HEALTHY AGING AND COGNITIVE DECLINE USING PROTEOFORM SIGNATURES FROM PAIRED CSF AND PLASMA
Abstract
Aims
While aging remains the most significant risk factor for Alzheimer’s disease (AD), the biological pathways that are altered in healthy aging vs. pathologic aging leading to neurodegeneration remain to be elucidated. Here, we seek to address this unmet need by applying a novel mass spectrometry-based discovery workflow. To unveil potential aging-related factors, we analyzed matched plasma and CSF proteomes between healthy aging and cognitive perturbations associated to the development of Alzheimer’s disease in its earliest phase.
Methods
Matched CSF and plasma samples were obtained from individuals at the same visit. Samples were collected from young control subjects (n= 53), subjects with mild cognitive impairment (MCI) (n = 40), age-matched healthy control subjects (n = 40) and subjects with autopsy-proven Alzheimer’s disease (n = 21). The plasma and CSF samples were subsequently processed to tryptic peptides and analyzed using a Thermo Scientific Orbitrap Exploris 480 equipped with a FAIMS Pro device. Clinical biomarkers (abeta40, abeta42, t-tau, ptau181) were assessed using the Lumipulse G1200 system.
Results
Using our optimized discovery workflow, we analyzed the above-described cohort of 133 matched plasma and CSF pairs and 21 additional CSF samples. This resulted in more than 50000 quantified peptides (associated with > 4000 proteins) in CSF and plasma. The correlative analyses of paired CSF and plasma samples allowed us to assess blood-brain barrier integrity in healthy and early pathological aging.
Conclusions
Harnessing the power of the latest advancement in mass spectrometry-based technique, we generated a comprehensive and quantitative map of proteoforms linked to healthy and pathological aging.
