
Moderator of 1 Session
Presenter of 1 Presentation
DATA-DRIVEN SUBTYPING AND POST HOC ANALYSIS OF AZD0530 (FYN) TRIAL
Abstract
Aims
The high failure rate of clinical trials in AD, coupled with increasing evidence of phenotypic and temporal heterogeneity in observational cohorts, suggests that traditional inclusion criteria may be inadequate. Computational disease progression models can unravel this heterogeneity to potentially select a homogeneous cohort with increased sensitivity to treatment effect. This study investigated whether such a model can identify and characterize heterogeneity within the phase 2a “FYN” clinical trial (NCT02167256).
Methods
Subtype and Stage Inference (SuStaIn) was used to learn subtypes of cumulative abnormality in regional brain volumes. After training on ADNI data (N=443), the model was deployed to assign subtype and stage to FYN data (N=159) at screening. A post hoc subgroup analysis was performed in these subgroups following the trial protocol. The primary outcome was brain glucose uptake (18F-FDG PET) over 52 weeks, with MRI and neuropsychological tests as secondary outcomes.
Results
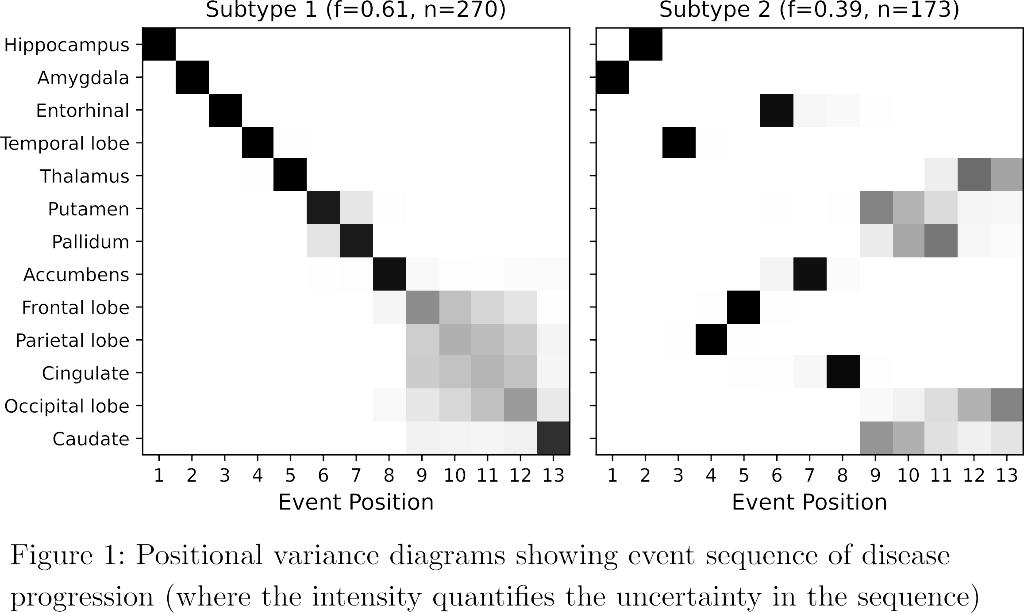
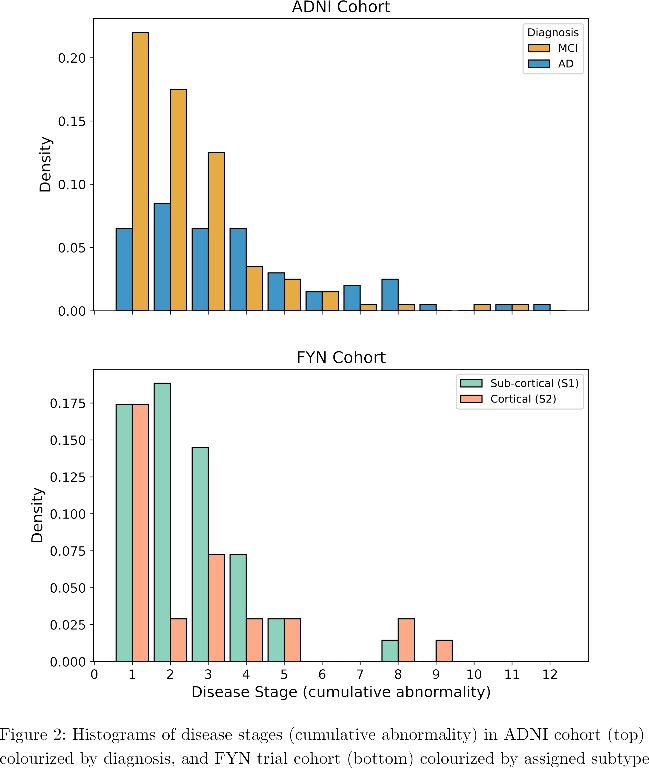
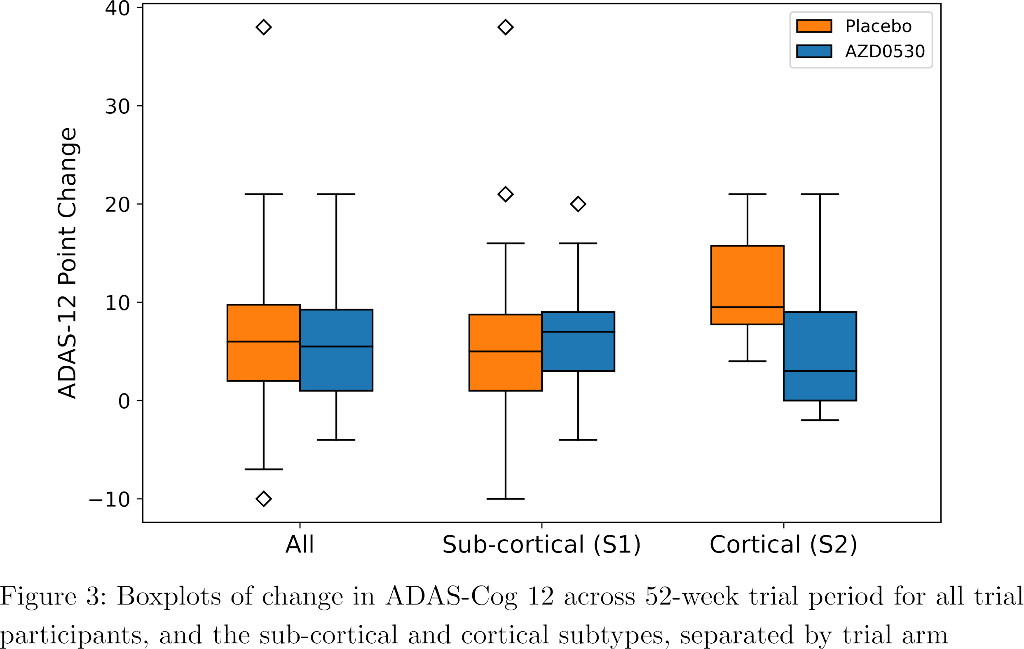
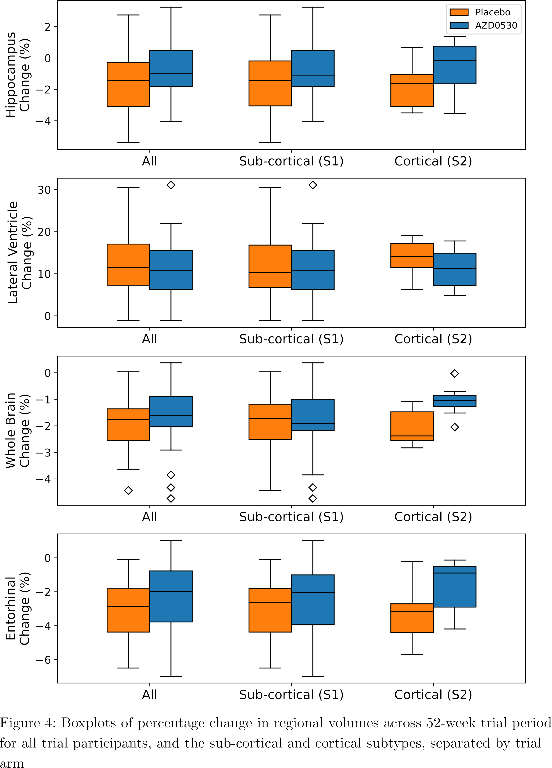
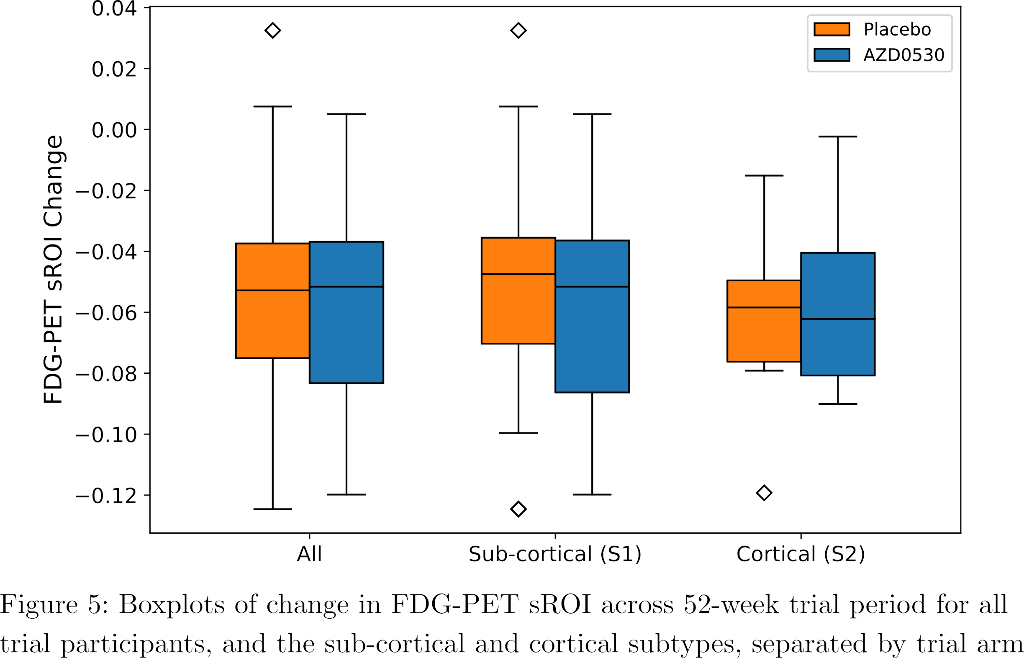
SuStaIn identified a subcortical (S1) and cortical (S2) subtype in ADNI (Figure 1), distinguished by regions involved earlier in each progression. The cortical subtype exhibited greater cognitive impairment (t-test, ADAS-13, p<0.001). The model revealed similar heterogeneity in FYN (Figure 2) and the cortical subtype (S2) showed potential treatment benefit in both secondary outcomes: ADAS-Cog-12 (Figure 3) and vMRI measures (Figure 4), but not in the primary outcome (Figure 5).
Conclusions
Our model characterized phenotypic and temporal heterogeneity in the AZD0530 trial cohort, and identified potential treatment effect in a post hoc subgroup, although the small sample size compels further investigation such as in Phase 3 trials.
