Shorena Janelidze, Sweden
Lund University Clinical Memory Research Unit, Department of Clinical Sciences MalmöAuthor Of 9 Presentations
PLASMA AMYLOID, P-TAU217, NFL, AND GFAP AS BIOMARKERS OF AMYLOID PATHOLOGY IN COGNITIVELY HEALTHY INDIVIDUALS
Abstract
Aims
To study if the accuracy of blood amyloid-β (Aβ) to detect Alzheimer disease (AD) at early stages could be improved by other blood biomarkers including phospho-tau217 (P-tau217), neurofilament light (NfL) and glial fibrillary acidic protein (GFAP).
Methods
We measured plasma Aβ42/Aβ40 (Araclon mass spectrometry [MS] or Euroimmun ELISA [EL]), Ptau-217 (Lilly assay), NfL (Simoa assay) and GFAP (Simoa assay) in cognitively unimpaired elderly from the Swedish BioFINDER-1 (n=242) and BioFINDER-2 (n=338) studies. In BioFINDER-1, out of 239 individuals followed longitudinally, 12 converted to AD dementia. CSF Aβ42/Aβ40 measures were available in all study participants, 568 individuals underwent Aβ-PET.
Results
In BioFINDER-1, plasma Aβ42/Aβ40MS identified individuals with abnormal CSF Aβ status with AUC of 0.79 (AIC=260). We observed a better performance (higher AUC and lower AIC) for models including plasma Aβ42/Aβ40MS and P-tau217 (AUC=0.83, AIC=230) or plasma Aβ42/Aβ40MS, P-tau217 and GFAP (AUC=0.85, AIC=224). In BioFINDER-2, the model with plasma Aβ42/Aβ40EL and P-tau217 as predictors provided better fit (AUC=0.84, AIC=319) for CSF Aβ status than plasma Aβ42/Aβ40EL alone (AUC=0.74, AIC=354). The results when using Aβ-PET instead of CSF Aβ status were very similar. In BioFINDER-1, a combination of plasma Aβ42/Aβ40MS and P-tau217 (AUC=0.82, AIC=88) better modelled conversion to AD dementia than plasma Aβ42/Aβ40MS (AUC=0.79, AIC=90). There was no further improvement when adding plasma NfL to the models.
Conclusions
The accuracy of blood test to detect preclinical cerebral Aβ pathology could be improved by combining plasma measurements of Aβ42/Aβ40, Ptau-217 and potentially GFAP.
PLASMA BIOMARKERS OF ALZHEIMER’S DISEASE PREDICT FUTURE COGNITIVE STATUS IN THE COGNITIVELY UNIMPAIRED ELDERLY
Abstract
Aims
Plasma biomarkers of amyloid-β (Aβ), tau, and neurodegeneration can accurately predict the risk of developing Alzheimer’s disease (AD) dementia in individuals with mild cognitive impairment (MCI), but their effectiveness in the cognitively unimpaired (CU) elderly population is unknown.
Methods
A total of 435 CU individuals were analyzed from the Swedish BioFINDER study with an average age of 72.5 years (range = [60, 88]). We tested the combined ability of plasma Aβ42/Aβ40, tau phosphorylated at threonine 217 (P-tau217), and neurofilament light (NfL) to predict (a) continuous four-year decline in Mini Mental State Examination (MMSE) scores, (b) which individuals remained cognitively stable (< 2 points decline). Plasma biomarkers were compared to a basic model which included only age, sex, and education.
Results
Adding plasma biomarkers to a basic demographics model significantly improved the prediction of continuous four-year MMSE decline (P=0.001) with P-tau217 and NfL having a significant effect (P=0.013 and 0.008, respectively). Adding plasma biomarkers also significantly improved prediction of cognitively stable individuals (P=0.0004), with P-tau217 and NfL again both having a significant effect (P=0.015 and 0.036, respectively). Figure 1 shows the relationship between biomarker levels and four-year change in MMSE (shown as baseline MMSE - four-year MMSE; positive values indicate worsening cognition).
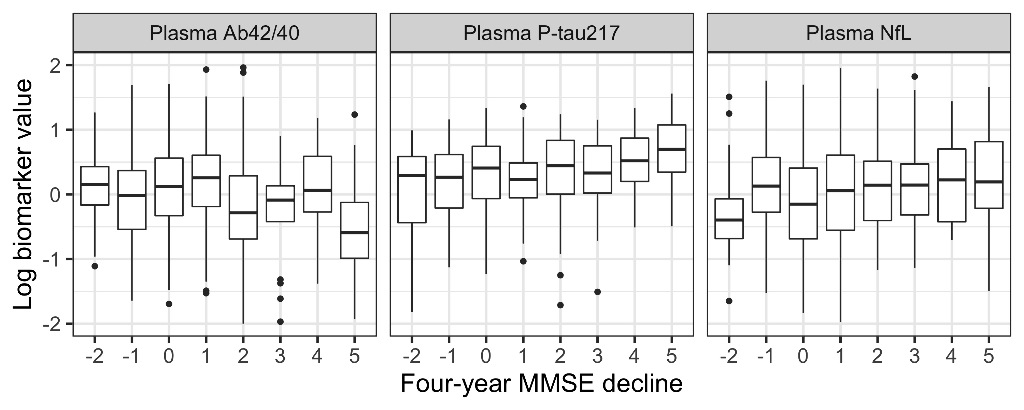
Conclusions
Plasma biomarkers of AD add significant prognostic information for the future cognitive status of elderly individuals without cognitive impairment, particularly for individuals at highest risk of cognitive decline. This further motivates their use in a screening context.
REMOTE MOBILE APP-BASED MEMORY ASSESSMENTS REFLECT TRADITIONAL MEMORY MEASURES AND ARE SENSITIVE TO MEASURES OF TAU PATHOLOGY
Abstract
Aims
The medial temporal lobe is particularly affected by AD pathology. One of the earliest anatomical sites where tau pathology can be detected is the transentorhinal region which has been associated with mnemonic discrimination of similar objects. Recent studies showed that object mnemonic discrimination was associated with fluid and imaging measures of tau pathology. Here we set out to evaluate the relationship of an adaptation of this memory task for mobile devices in an unsupervised setting with neuropsychological measures and biomarkers of tau pathology.
Methods
59 non-demented individuals of the Swedish BioFINDER study (34% β-amyloid positive, mean age 62yrs, 59% female) participated in on-site memory assessments and underwent MRI and [18F]RO948 tau-PET scans. In addition, participants completed up to 12 remote memory tests using their own mobile devices. Here we report memory performance as a mean estimate across the first two remote sessions.
Results
Remote memory assessments correlated with computerized on-site assessments using a similar task for object-and-scene memory (Berron et al., 2018) (r=0.72,p<.001) as well as with delayed word recall performance (r=-0.57,p<.001). Remote object but not scene memory showed a significant relationship with tau-PET SUVr in the transentorhinal region (β=-0.11,SE=0.05,p=.039) and plasma pTau217 levels (β=-0.04,SE=0.017,p=.047). Finally, remote object memory was lower in individuals with thinner cortex in the transentorhinal region (β=0.31,SE=0.11,p=.009).
Conclusions
Our results demonstrate that remote and unsupervised memory assessments via mobile devices are (i) comparable to supervised computerized on-site testing, (ii) show a relationship with traditional neuropsychological measures for memory, and (iii) are sensitive to underlying tau pathology.
LIVE DISCUSSION
PLASMA GLIAL FIBRILLARY ACIDIC PROTEIN PREDICTS AMYLOID STATUS AND FUTURE CONVERSION TO ALZHEIMER’S DISEASE IN A MILD COGNITIVE IMPAIRMENT LONGITUDINAL COHORT
Abstract
Aims
Astrogliosis in response to amyloid-beta (Aβ) plaques is an early feature of Alzheimer's disease (AD). Glial fibrillary acidic protein (GFAP) is expressed in astrocytes and is increased in CSF in AD. Studies on plasma GFAP as AD biomarker are few and not longitudinal. Our aim was to evaluate plasma GFAP as potential biomarker for Aβ status and for future development of AD dementia.
Methods
161 subjects with a baseline clinical diagnosis of mild cognitive impairment (MCI) were included, genotyped for APOE, followed for 4.7 years (average) and assessed for conversion to AD at follow-up. Plasma was collected at baseline and follow-up. GFAP was measured with Simoa GFAP Discovery kit for SR-X (Quanterix). Aß positivity (Aß+) was defined as CSF Aβ42/40 <0.07 (cut-off calculated with Youden index within the cohort).
Results
Baseline GFAP was increased in Aβ+ MCI patients (p<0.0001). Plasma GFAP could predict Aβ+ status (p<0.0001, AIC=184.3, AUC=0.787, sensitivity=73%, specificity=75%). Accuracy was increased by combining plasma GFAP and APOE genotype (p<0.0001, AIC 154.7, AUC=0.859). Plasma GFAP could also predict subsequent development of AD dementia (p<0.0001, AIC=154.4, AUC=0.836, sensitivity=72%, specificity=85%). Predictive accuracy of future AD dementia was improved by combining plasma GFAP with APOE genotype and age (p<0.0001, AIC=140, AUC=0.864). Longitudinal slopes showed a significant increase of plasma GFAP over time in Aβ+ MCI compared to Aβ- (p<0.0001) and in subjects later diagnosed with AD compared to those that remained clinically stable (stable Aβ-:p<0.0001; stable Aβ+:p=0.049).
Conclusions
Plasma GFAP is strongly associated to Aβ status and is a good predictor of clinical evolution to AD.
MARKERS OF SMALL VESSEL DISEASE ARE ASSOCIATED WITH CSF BIOMARKERS OF NEUROINFLAMMATION AND CEREBROVASCULAR DYSFUNCTION IN NEURODEGENERATIVE DISEASES
Abstract
Aims
Different biomarkers of neuroinflammation and cerebrovascular dysfunction are thought to be associated with neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), even during their preclinical and prodromal stages. However, little is known so far about associations between inflammatory markers and white matter lesions (WML) detected with magnetic resonance imaging (MRI).
Methods
We included 582 cognitively unimpaired (CU) elderly, 279 patients with mild cognitive impairment (MCI), and 175 PD patients. CSF samples were analyzed for interleukin (IL)–6, IL-7, IL-8, IL-15, IL-16, interferon-gamma induced protein-10 (IP-10), monocyte chemoattractant protein 1 (MCP1), intercellular adhesion molecule 1(ICAM-1), vascular adhesion molecule 1 (VCAM-1), placental growth factor (PIGF), and fms-related tyrosine kinase 1 (Flt-1) as well as vascular endothelial growth factor (VEGF). WML volumes were determined from MRI for all subjects.
Results
In CU, more WML was associated with higher levels of PIGF (β=0.007, p<0.001) and lower levels of sFLT1 (β=-0.018, p<0.001), whereas in MCI more WML volume was associated with higher levels of IL-16 (β=0.118, p<0.001), higher levels of PIGF (β=0.007, p<0.001), and higher levels of VEGF (β=0.105, p=0.003). Associations were still significant after corrections for multiple comparisons. In PD higher levels of WML were associated with VEGF (β=0.374, p=0.007) and PLGF (β=0.321, p=0.019), however those associations were not significant after correction for multiple comparison.
Conclusions
CSF markers of vascular system, such as VEGF and PLGF, are associated with cerebrovascular injury in prodromal AD and PD.
PLASMA PHOSPHO-TAU IDENTIFIES ALZHEIMER’S CO-PATHOLOGY IN PATIENTS WITH LEWY BODY DISEASE WITH DEMENTIA
Abstract
Aims
To investigate whether plasma phospho-tau217 (P-tau217) and phospho-tau181 (P-tau181) can detect Alzheimer’s disease (AD) co-pathology in patients with dementia with Lewy bodies and Parkinson’s disease with dementia (i.e. Lewy body disease with dementia).
Methods
In this cross-sectional study we investigated the plasma levels of P-tau217 and P-tau181 in 35 patients with dementia with Lewy bodies or Parkinson’s disease with dementia, recruited as part of the Swedish BioFINDER2 study. All patients also underwent tau-PET imaging using 18F-RO948, and cerebrospinal fluid (CSF) was analyzed for P-tau217, P-tau181 and β-amyloid42/40 (Aβ42/Aβ40), biomarkers that reliably detect AD pathology in vivo. Abnormal β-amyloid status was defined as CSF Aβ42/Aβ40 <0.752, determined using mixture modeling.
Results
Plasma P-tau217 correlated with plasma P-tau181 (rs=0.68, p<0.001), CSF P-tau217 (rs=0.68, p<0.001) and negatively with CSF Aβ42/Aβ40 (rs=-0.52, p=0.001). Plasma P-tau181 correlated with CSF P-tau181 (rs=0.55, p<0.001). Both plasma P-tau217 and plasma P-tau181 correlated with 18F-RO948 retention in the temporal meta ROI corresponding to Braak stage I-IV (rs=0.57, p<0.001 and rs=0.66, p<0.001, respectively). Additionally, plasma P-tau217 and plasma P-tau181 predicted abnormal tau-PET status in the temporal meta ROI (AUC 0.84 and 0.78, respectively) as well as abnormal CSF β-amyloid status (AUC 0.88 and 0.81, respectively).
Conclusions
Plasma P-tau might be a useful marker for the detection of AD co-pathology in Lewy body disease with dementia, and could be used for stratification of patients in clinical trials. Further studies are needed to determine whether plasma P-tau provides important prognostic information in patients with Lewy body disease.
PREDICTION OF ALZHEIMER’S DISEASE DEMENTIA USING PLASMA P-TAU217 IN COMBINATION WITH OTHER NON-INVASIVE MEASURES: LONGITUDINAL STUDY FROM THE BIOFINDER AND ADNI COHORTS
Abstract
Aims
To examine the accuracy of plasma phospho-tau217 (P-tau217) for predicting Alzheimer’s disease dementia (AD) when combined with other non-invasive biomarkers. The accuracy was compared to the clinical diagnostic prediction.
Methods
328 participants with subjective cognitive decline (SCD, n=155) or mild cognitive impairment (MCI, n=173) at baseline were included from the Swedish BioFINDER study. Plasma P-tau217, cognitive tests, demographics, plasma Aβ42/40, plasma neurofilament light (NfL), and MRI (AD-signature cortical thickness) were examined using logistic regression models with conversion to AD at 2, 4 and 6 years, respectively, as outcomes. Model selection and area under the ROC curve (AUC) were validated in 531 participants with SCD or MCI from the ADNI study using plasma P-tau181 instead of P-tau217.
Results
Plasma P-tau217 predicted conversion to AD with an AUC of 0.78 (95%CI 0.72-0.85) at 2 years, 0.82 (0.77-0.88) at 4 years and 0.85 (0.80-0.90) at 6 years. 2-year prediction of AD was improved by adding MRI, memory, executive function, and gender to P-tau217 (0.88, 0.83-0.94). 4-year prediction was improved by adding MRI, memory, and executive function (0.90, 0.86-0.93). 6-year prediction was improved by adding plasma Aβ42/40 and NfL, MRI, and memory (0.91, 0.87-0.94). In ADNI, similar models were selected with similar accuracies. The diagnostic prediction of memory clinic physicians blinded to biomarker data were inferior to all models (AUCs 0.68-0.73).
Conclusions
Plasma P-tau can accurately predict future AD, better than a clinical diagnostic prediction. The accuracy can be improved further by adding brief cognitive tests and MRI, and to a lesser extent plasma Aβ42/40 and NfL.
PLASMA P-TAU217 PREDICTS LONGITUDINAL AMYLOID ACCUMULATION, TAU BURDEN, BRAIN ATROPHY AND COGNITIVE DECLINE IN EARLY ALZHEIMER’S DISEASE
Abstract
Aims
It is currently unclear whether plasma biomarkers can be used as prognostic tools for Alzheimer’s disease (AD). Here we address this question by examining plasma amyloid-β 42/40 (Aβ42/40), phosphorylated-tau 181 (P-tau181), phosphorylated-tau 217 (P-tau217) and neurofilament light (NfL) in non-demented individuals who underwent longitudinal amyloid and tau positron emission tomography (PET), magnetic resonance imaging (MRI) and cognitive testing.
Methods
Blood was collected at baseline from all participants to determine the levels of Aβ42/40, P-tau181, P-tau217 and NfL. All subjects underwent longitudinal 18F-RO948 PET, structural MRI and cognitive assessment. In addition, a subsample also had longitudinal 18F-flutemetamol PET scans. Linear mixed effects models and voxel-wise analyses were applied to assess the relationship between plasma biomarkers and longitudinal changes in imaging and cognition.
Results
Our results show that plasma P-tau217 predicts increases in amyloid (p=0.017) and tau (p=0.004) PET, medial temporal atrophy (p<0.001) and global cognitive decline (p=0.005). The results for the other plasma markers were more variable, with plasma Aβ42/40 predicting amyloid (p=0.002) and tau (p=0.003) PET signal increases as well as cognition (p=0.020), whereas P-tau181 predicts hippocampal atrophy (p=0.008) and cognitive decline (p=0.003), and finally NfL predicts atrophy (p=0.001) and tau PET increases (p=0.020). The comparison between the models showed that the ones that included P-tau217 as a predictor had a significantly better fit to the data as indicated by lower Bayesian Information Criterion values.
Conclusions
These findings suggest that plasma P-tau217 could be useful in clinical trials to determine whether an individual is on the pathophysiological pathway of AD.
Presenter of 2 Presentations
LIVE DISCUSSION
PLASMA AMYLOID, P-TAU217, NFL, AND GFAP AS BIOMARKERS OF AMYLOID PATHOLOGY IN COGNITIVELY HEALTHY INDIVIDUALS
Abstract
Aims
To study if the accuracy of blood amyloid-β (Aβ) to detect Alzheimer disease (AD) at early stages could be improved by other blood biomarkers including phospho-tau217 (P-tau217), neurofilament light (NfL) and glial fibrillary acidic protein (GFAP).
Methods
We measured plasma Aβ42/Aβ40 (Araclon mass spectrometry [MS] or Euroimmun ELISA [EL]), Ptau-217 (Lilly assay), NfL (Simoa assay) and GFAP (Simoa assay) in cognitively unimpaired elderly from the Swedish BioFINDER-1 (n=242) and BioFINDER-2 (n=338) studies. In BioFINDER-1, out of 239 individuals followed longitudinally, 12 converted to AD dementia. CSF Aβ42/Aβ40 measures were available in all study participants, 568 individuals underwent Aβ-PET.
Results
In BioFINDER-1, plasma Aβ42/Aβ40MS identified individuals with abnormal CSF Aβ status with AUC of 0.79 (AIC=260). We observed a better performance (higher AUC and lower AIC) for models including plasma Aβ42/Aβ40MS and P-tau217 (AUC=0.83, AIC=230) or plasma Aβ42/Aβ40MS, P-tau217 and GFAP (AUC=0.85, AIC=224). In BioFINDER-2, the model with plasma Aβ42/Aβ40EL and P-tau217 as predictors provided better fit (AUC=0.84, AIC=319) for CSF Aβ status than plasma Aβ42/Aβ40EL alone (AUC=0.74, AIC=354). The results when using Aβ-PET instead of CSF Aβ status were very similar. In BioFINDER-1, a combination of plasma Aβ42/Aβ40MS and P-tau217 (AUC=0.82, AIC=88) better modelled conversion to AD dementia than plasma Aβ42/Aβ40MS (AUC=0.79, AIC=90). There was no further improvement when adding plasma NfL to the models.
Conclusions
The accuracy of blood test to detect preclinical cerebral Aβ pathology could be improved by combining plasma measurements of Aβ42/Aβ40, Ptau-217 and potentially GFAP.



