Welcome to the AD/PD™ 2021 Interactive Program
The congress will officially run on Central European Time (CET) - Barcelona Time
To convert the congress times to your local time Click Here
Icons Legend: ![]()
 - Live Session |
- Live Session |  - On Demand Session |
- On Demand Session |  - On Demand with Live Q&A
- On Demand with Live Q&A
The viewing of sessions, cannot be accessed from this conference calendar. All sessions are accessible via the Main Lobby.
FOLLOWING THE LIVE DISCUSSION, THE RECORDING WILL BE AVAILABLE IN THE ON-DEMAND SECTION OF THE AUDITORIUM.
PREDICTION OF ALZHEIMER’S DISEASE DEMENTIA USING PLASMA P-TAU217 IN COMBINATION WITH OTHER NON-INVASIVE MEASURES: LONGITUDINAL STUDY FROM THE BIOFINDER AND ADNI COHORTS
Abstract
Aims
To examine the accuracy of plasma phospho-tau217 (P-tau217) for predicting Alzheimer’s disease dementia (AD) when combined with other non-invasive biomarkers. The accuracy was compared to the clinical diagnostic prediction.
Methods
328 participants with subjective cognitive decline (SCD, n=155) or mild cognitive impairment (MCI, n=173) at baseline were included from the Swedish BioFINDER study. Plasma P-tau217, cognitive tests, demographics, plasma Aβ42/40, plasma neurofilament light (NfL), and MRI (AD-signature cortical thickness) were examined using logistic regression models with conversion to AD at 2, 4 and 6 years, respectively, as outcomes. Model selection and area under the ROC curve (AUC) were validated in 531 participants with SCD or MCI from the ADNI study using plasma P-tau181 instead of P-tau217.
Results
Plasma P-tau217 predicted conversion to AD with an AUC of 0.78 (95%CI 0.72-0.85) at 2 years, 0.82 (0.77-0.88) at 4 years and 0.85 (0.80-0.90) at 6 years. 2-year prediction of AD was improved by adding MRI, memory, executive function, and gender to P-tau217 (0.88, 0.83-0.94). 4-year prediction was improved by adding MRI, memory, and executive function (0.90, 0.86-0.93). 6-year prediction was improved by adding plasma Aβ42/40 and NfL, MRI, and memory (0.91, 0.87-0.94). In ADNI, similar models were selected with similar accuracies. The diagnostic prediction of memory clinic physicians blinded to biomarker data were inferior to all models (AUCs 0.68-0.73).
Conclusions
Plasma P-tau can accurately predict future AD, better than a clinical diagnostic prediction. The accuracy can be improved further by adding brief cognitive tests and MRI, and to a lesser extent plasma Aβ42/40 and NfL.
PLASMA AMYLOID, P-TAU217, NFL, AND GFAP AS BIOMARKERS OF AMYLOID PATHOLOGY IN COGNITIVELY HEALTHY INDIVIDUALS
Abstract
Aims
To study if the accuracy of blood amyloid-β (Aβ) to detect Alzheimer disease (AD) at early stages could be improved by other blood biomarkers including phospho-tau217 (P-tau217), neurofilament light (NfL) and glial fibrillary acidic protein (GFAP).
Methods
We measured plasma Aβ42/Aβ40 (Araclon mass spectrometry [MS] or Euroimmun ELISA [EL]), Ptau-217 (Lilly assay), NfL (Simoa assay) and GFAP (Simoa assay) in cognitively unimpaired elderly from the Swedish BioFINDER-1 (n=242) and BioFINDER-2 (n=338) studies. In BioFINDER-1, out of 239 individuals followed longitudinally, 12 converted to AD dementia. CSF Aβ42/Aβ40 measures were available in all study participants, 568 individuals underwent Aβ-PET.
Results
In BioFINDER-1, plasma Aβ42/Aβ40MS identified individuals with abnormal CSF Aβ status with AUC of 0.79 (AIC=260). We observed a better performance (higher AUC and lower AIC) for models including plasma Aβ42/Aβ40MS and P-tau217 (AUC=0.83, AIC=230) or plasma Aβ42/Aβ40MS, P-tau217 and GFAP (AUC=0.85, AIC=224). In BioFINDER-2, the model with plasma Aβ42/Aβ40EL and P-tau217 as predictors provided better fit (AUC=0.84, AIC=319) for CSF Aβ status than plasma Aβ42/Aβ40EL alone (AUC=0.74, AIC=354). The results when using Aβ-PET instead of CSF Aβ status were very similar. In BioFINDER-1, a combination of plasma Aβ42/Aβ40MS and P-tau217 (AUC=0.82, AIC=88) better modelled conversion to AD dementia than plasma Aβ42/Aβ40MS (AUC=0.79, AIC=90). There was no further improvement when adding plasma NfL to the models.
Conclusions
The accuracy of blood test to detect preclinical cerebral Aβ pathology could be improved by combining plasma measurements of Aβ42/Aβ40, Ptau-217 and potentially GFAP.
PLASMA PHOSPHO-TAU IDENTIFIES ALZHEIMER’S CO-PATHOLOGY IN PATIENTS WITH LEWY BODY DISEASE WITH DEMENTIA
Abstract
Aims
To investigate whether plasma phospho-tau217 (P-tau217) and phospho-tau181 (P-tau181) can detect Alzheimer’s disease (AD) co-pathology in patients with dementia with Lewy bodies and Parkinson’s disease with dementia (i.e. Lewy body disease with dementia).
Methods
In this cross-sectional study we investigated the plasma levels of P-tau217 and P-tau181 in 35 patients with dementia with Lewy bodies or Parkinson’s disease with dementia, recruited as part of the Swedish BioFINDER2 study. All patients also underwent tau-PET imaging using 18F-RO948, and cerebrospinal fluid (CSF) was analyzed for P-tau217, P-tau181 and β-amyloid42/40 (Aβ42/Aβ40), biomarkers that reliably detect AD pathology in vivo. Abnormal β-amyloid status was defined as CSF Aβ42/Aβ40 <0.752, determined using mixture modeling.
Results
Plasma P-tau217 correlated with plasma P-tau181 (rs=0.68, p<0.001), CSF P-tau217 (rs=0.68, p<0.001) and negatively with CSF Aβ42/Aβ40 (rs=-0.52, p=0.001). Plasma P-tau181 correlated with CSF P-tau181 (rs=0.55, p<0.001). Both plasma P-tau217 and plasma P-tau181 correlated with 18F-RO948 retention in the temporal meta ROI corresponding to Braak stage I-IV (rs=0.57, p<0.001 and rs=0.66, p<0.001, respectively). Additionally, plasma P-tau217 and plasma P-tau181 predicted abnormal tau-PET status in the temporal meta ROI (AUC 0.84 and 0.78, respectively) as well as abnormal CSF β-amyloid status (AUC 0.88 and 0.81, respectively).
Conclusions
Plasma P-tau might be a useful marker for the detection of AD co-pathology in Lewy body disease with dementia, and could be used for stratification of patients in clinical trials. Further studies are needed to determine whether plasma P-tau provides important prognostic information in patients with Lewy body disease.
PLASMA P-TAU 181, P-TAU 217 AND OTHER BLOOD-BASED ALZHEIMER’S DISEASE BIOMARKERS IN A MULTI-ETHNIC, COMMUNITY STUDY
Abstract
Aims
Blood-based Alzheimer’s disease (AD) biomarkers provide opportunities for community studies and across ethnic groups.
We investigated blood biomarker concentrations in the Washington Heights, Inwood, Columbia Aging Project (WHICAP), a
multi-ethnic community study of aging and dementia. The goal was to determine the effectiveness of state-of-the-art ADrelated
plasma biomarkers, including Aβ40 and ββ42 as markers of amyloid pathology, total tau and neurofilament light
chain (NfL) as markers of neurodegeneration, and phospho-tau (P-tau) 181 and 217 as markers of tau pathology. We
compared plasma biomarker concentrations between clinically- and pathologically-defined diagnostic groups and
examined differences by race/ethnicity groups.
Methods
We measured plasma Aβ40, Aβ42,T-tau, P-tau181 and P-tau217, and neurofilament light chain (NfL) in 113 autopsied
participants, (29% with high AD neuropathological changes) and in 300 clinically evaluated individuals (42% with clinical
AD). Receiver operating characteristics were used to evaluate each biomarker. We also investigated biomarkers as
predictors of incident clinical AD.
Results
P-tau181, P-tau217 and NfL concentrations were elevated in pathologically diagnosed AD and to a lesser extent in
clinically diagnosed AD. Decreased Aβ42/Aβ40 ratio and increased P-tau217 and P-tau181 were associated with
subsequent AD diagnosis in a follow-up of previously unaffected individuals.
Conclusions
Blood-based AD biomarker concentrations of the Aβ42/Aβ40 ratio, P-tau217 and P-tau181 are associated with
pathological and clinical diagnoses and can predict future development of clinical AD, providing evidence that they can be
incorporated into multi-ethnic, community-based studies.
P-TAU235: A NOVEL BIOMARKER OF EMERGING AD PATHOLOGY
Abstract
Aims
Recently, novel immunoassays targeting phosphorylated tau species in CSF and blood (e.g., p-tau181 and p-tau217) have shown high specificity in identifying Alzheimer’s disease (AD) pathology. However, there remains a need for biomarker to detect non-AD tauopathies. Therefore, our aim was to identify other phosphorylation sites which could serve as novel biomarkers capable of discriminating non-AD tauopathies from AD.
Methods
Novel tau phosphorylations were identified through immunoprecipitation (IP) followed by mass spectrometry (MS) in soluble protein extracts from brain frontal grey matter in a cohort of control cases (n=10), AD (n=10) and prominent non-AD tauopathies (n=30). Based on the IP-MS results, we developed an ultrasensitive immunoassay targeting a novel tau phosphorylation site at Ser235 using Simoa technology. We assessed the performance of our novel p-tau235 assay in a pilot study with CSF samples from AD patients (n=19) and controls (n=20), defined by the core AD CSF biomarkers (t-tau, p-tau181 and Abeta).
Results
 In the pilot CSF cohort, our novel immunoassay showed that p-tau235 was significantly increased in AD when compared to controls (p˂0.001, Mann-Whitney) (Figure-1). Moreover, p-tau235 showed a high accuracy in discriminating AD from control individuals (AUC=88.5%, 95%CI=78.2-98.7%) (Figure-2). CSF p-tau235 showed significant correlations with the core AD biomarkers t-tau (r=0.78), p-tau181 (r=0.73) and Abeta42 (r=-0.65), all at p˂0.001.
In the pilot CSF cohort, our novel immunoassay showed that p-tau235 was significantly increased in AD when compared to controls (p˂0.001, Mann-Whitney) (Figure-1). Moreover, p-tau235 showed a high accuracy in discriminating AD from control individuals (AUC=88.5%, 95%CI=78.2-98.7%) (Figure-2). CSF p-tau235 showed significant correlations with the core AD biomarkers t-tau (r=0.78), p-tau181 (r=0.73) and Abeta42 (r=-0.65), all at p˂0.001.
Conclusions
Although preliminary, the p-tau235 assay showed good performance identifying AD patients and showed high correlations with the core AD CSF biomarkers. Therefore, further results will assess the diagnostic performance of Ser235 in larger cohorts, which will include CSF from AD and non-AD tauopathies.
AΒ-DOWNSTREAM CSF BIOMARKERS ARE ASSOCIATED WITH GREY MATTER VOLUME AND CEREBRAL GLUCOSE CONSUMPTION IN THE EARLY STAGES OF THE ALZHEIMER’S CONTINUUM
Abstract
Aims
To describe associations between imaging markers of neurodegeneration and cerebrospinal fluid (CSF) biomarkers of pathophysiological pathways altered in Alzheimer's disease (AD).
Methods
We included 336 cognitively unimpaired participants (mean age[standard deviation]: 61.3[4.7] years) from the ALFA+ cohort with T1-weighted MRI, FDG-PET and eleven CSF biomarkers (NeuroToolKit, Roche Diagnostics). Projection to Latent Spaces was conducted to detect combinations of CSF biomarkers (components) that explained the maximal variance in imaging markers (GMv and FDG-uptake independently). We sought for associations between CSF components and imaging neurodegeneration markers across A/T stages and both in AD-sensitive regions and at the voxel level.
Results
Higher CSF sTREM2, mostly increased in A+T+, was associated with higher GMv in the inferior temporal cortex(Fig.1a). CSF t-tau, p-tau, and neurogranin, which progressively increased with more advanced A/T stages, were negatively associated to the structural AD-signature, but positively associated with GMv in other extensive regions(Fig.1b). A neurodegeneration(NfL,α-synuclein) and inflammation-related component(YKL-40, GFAP) was associated with lower GMv in AD-sensitive areas (Fig.1c). Higher levels of neuroinflammatory markers(GFAP,IL-6,s100b) were associated with higher GMv in the cerebellum (Fig.1d). FDG-uptake was negatively associated with CSF tau, neurogranin and α-synuclein in the medial frontal/parietal areas(Fig.1e), but positively in the insula and thalamus(Fig.1e). A component of markers of inflammation(IL-6,YKL-40) and neurodegeneration(NfL), mostly increased in A+T+, was associated with lower FDG-uptake in AD-sensitive regions(Fig.1f).
Conclusions
Multiple pathophysiological pathways are altered with the onset of AD pathology and are associated with imaging markers of neurodegeneration in the earliest stages of the Alzheimer's continuum.

PLASMA BIOMARKERS OF ALZHEIMER’S DISEASE PREDICT FUTURE COGNITIVE STATUS IN THE COGNITIVELY UNIMPAIRED ELDERLY
Abstract
Aims
Plasma biomarkers of amyloid-β (Aβ), tau, and neurodegeneration can accurately predict the risk of developing Alzheimer’s disease (AD) dementia in individuals with mild cognitive impairment (MCI), but their effectiveness in the cognitively unimpaired (CU) elderly population is unknown.
Methods
A total of 435 CU individuals were analyzed from the Swedish BioFINDER study with an average age of 72.5 years (range = [60, 88]). We tested the combined ability of plasma Aβ42/Aβ40, tau phosphorylated at threonine 217 (P-tau217), and neurofilament light (NfL) to predict (a) continuous four-year decline in Mini Mental State Examination (MMSE) scores, (b) which individuals remained cognitively stable (< 2 points decline). Plasma biomarkers were compared to a basic model which included only age, sex, and education.
Results
Adding plasma biomarkers to a basic demographics model significantly improved the prediction of continuous four-year MMSE decline (P=0.001) with P-tau217 and NfL having a significant effect (P=0.013 and 0.008, respectively). Adding plasma biomarkers also significantly improved prediction of cognitively stable individuals (P=0.0004), with P-tau217 and NfL again both having a significant effect (P=0.015 and 0.036, respectively). Figure 1 shows the relationship between biomarker levels and four-year change in MMSE (shown as baseline MMSE - four-year MMSE; positive values indicate worsening cognition).
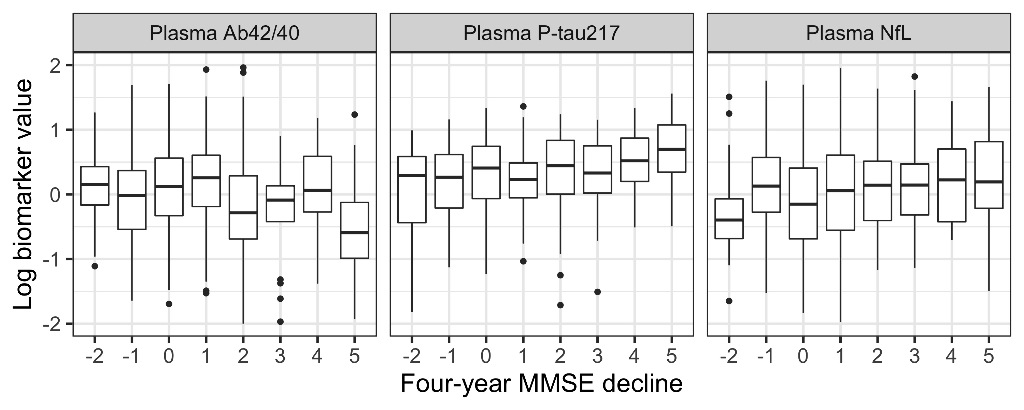
Conclusions
Plasma biomarkers of AD add significant prognostic information for the future cognitive status of elderly individuals without cognitive impairment, particularly for individuals at highest risk of cognitive decline. This further motivates their use in a screening context.
DIAGNOSTIC PERFORMANCE OF BLOOD BIOMARKERS FOR ALZHEIMER’S DISEASE IN AN UNSELECTED MEMORY CLINIC COHORT
Abstract
Aims
To compare the diagnostic performance of plasma biomarkers for Alzheimer’s disease (AD) diagnosis in a diverse memory clinic setting.
Methods
We compared two recently developed plasma p-tau Single molecule array assays (p-tau181 and p-tau231), plasma neurofilament light (NfL) and plasma amyloid ratio (Aβ42/40) in diagnosing AD and identifying amyloid positivity, in 310 subjects: 32 controls, 60 with AD related mild cognitive impairment (MCI) and 104 with AD-dementia, 31 non-AD dementia’s (Lewy body dementia, frontotemporal dementia and vascular dementia), and 83 with non-AD related MCI. Patients were recruited in a memory clinic setting (Hospital Lariboisière, Paris, France).
Results
P-tau181, p-tau231 and NfL demonstrated high accuracy in identifying AD compared to controls (p-tau181: AUC=88.7%; p-tau231: AUC=85.4%; NFL: AUC=84.4%), performing better than amyloid ratio (AUC=70.2%). P-tau231 and p-tau181 also distinguished AD-MCI from controls (p-tau181: AUC=78.6%; p-tau231: AUC=78.4%). Both tau biomarkers separated amyloid+ from amyloid– patients (p-tau181: AUC=80.48%; p-tau231: AUC=80.31%), with higher accuracy than NfL (AUC=65.8%) and amyloid ratio (67.8%). P-tau181 and p-tau231 also distinguished AD from non-AD dementia (p-tau231: AUC=82.72%; p-tau181: AUC=81.22%), as opposed to NfL (AUC=57.78%).
Conclusions
Plasma p-tau181 and p-tau231 accurately identified AD at MCI and dementia stages of the disease, and efficiently distinguished AD dementia from non-AD dementia in a clinical setting. These could be simple and accessible tests for AD screening and diagnosis.


