
Sebastian Palmqvist, Sweden
Lund University Clinical Memory Research Unit, Department of Clinical Sciences MalmöAuthor Of 7 Presentations
MARKERS OF SMALL VESSEL DISEASE ARE ASSOCIATED WITH CSF BIOMARKERS OF NEUROINFLAMMATION AND CEREBROVASCULAR DYSFUNCTION IN NEURODEGENERATIVE DISEASES
Abstract
Aims
Different biomarkers of neuroinflammation and cerebrovascular dysfunction are thought to be associated with neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), even during their preclinical and prodromal stages. However, little is known so far about associations between inflammatory markers and white matter lesions (WML) detected with magnetic resonance imaging (MRI).
Methods
We included 582 cognitively unimpaired (CU) elderly, 279 patients with mild cognitive impairment (MCI), and 175 PD patients. CSF samples were analyzed for interleukin (IL)–6, IL-7, IL-8, IL-15, IL-16, interferon-gamma induced protein-10 (IP-10), monocyte chemoattractant protein 1 (MCP1), intercellular adhesion molecule 1(ICAM-1), vascular adhesion molecule 1 (VCAM-1), placental growth factor (PIGF), and fms-related tyrosine kinase 1 (Flt-1) as well as vascular endothelial growth factor (VEGF). WML volumes were determined from MRI for all subjects.
Results
In CU, more WML was associated with higher levels of PIGF (β=0.007, p<0.001) and lower levels of sFLT1 (β=-0.018, p<0.001), whereas in MCI more WML volume was associated with higher levels of IL-16 (β=0.118, p<0.001), higher levels of PIGF (β=0.007, p<0.001), and higher levels of VEGF (β=0.105, p=0.003). Associations were still significant after corrections for multiple comparisons. In PD higher levels of WML were associated with VEGF (β=0.374, p=0.007) and PLGF (β=0.321, p=0.019), however those associations were not significant after correction for multiple comparison.
Conclusions
CSF markers of vascular system, such as VEGF and PLGF, are associated with cerebrovascular injury in prodromal AD and PD.
PREDICTION OF ALZHEIMER’S DISEASE DEMENTIA USING PLASMA P-TAU217 IN COMBINATION WITH OTHER NON-INVASIVE MEASURES: LONGITUDINAL STUDY FROM THE BIOFINDER AND ADNI COHORTS
Abstract
Aims
To examine the accuracy of plasma phospho-tau217 (P-tau217) for predicting Alzheimer’s disease dementia (AD) when combined with other non-invasive biomarkers. The accuracy was compared to the clinical diagnostic prediction.
Methods
328 participants with subjective cognitive decline (SCD, n=155) or mild cognitive impairment (MCI, n=173) at baseline were included from the Swedish BioFINDER study. Plasma P-tau217, cognitive tests, demographics, plasma Aβ42/40, plasma neurofilament light (NfL), and MRI (AD-signature cortical thickness) were examined using logistic regression models with conversion to AD at 2, 4 and 6 years, respectively, as outcomes. Model selection and area under the ROC curve (AUC) were validated in 531 participants with SCD or MCI from the ADNI study using plasma P-tau181 instead of P-tau217.
Results
Plasma P-tau217 predicted conversion to AD with an AUC of 0.78 (95%CI 0.72-0.85) at 2 years, 0.82 (0.77-0.88) at 4 years and 0.85 (0.80-0.90) at 6 years. 2-year prediction of AD was improved by adding MRI, memory, executive function, and gender to P-tau217 (0.88, 0.83-0.94). 4-year prediction was improved by adding MRI, memory, and executive function (0.90, 0.86-0.93). 6-year prediction was improved by adding plasma Aβ42/40 and NfL, MRI, and memory (0.91, 0.87-0.94). In ADNI, similar models were selected with similar accuracies. The diagnostic prediction of memory clinic physicians blinded to biomarker data were inferior to all models (AUCs 0.68-0.73).
Conclusions
Plasma P-tau can accurately predict future AD, better than a clinical diagnostic prediction. The accuracy can be improved further by adding brief cognitive tests and MRI, and to a lesser extent plasma Aβ42/40 and NfL.
PLASMA P-TAU217 PREDICTS LONGITUDINAL AMYLOID ACCUMULATION, TAU BURDEN, BRAIN ATROPHY AND COGNITIVE DECLINE IN EARLY ALZHEIMER’S DISEASE
Abstract
Aims
It is currently unclear whether plasma biomarkers can be used as prognostic tools for Alzheimer’s disease (AD). Here we address this question by examining plasma amyloid-β 42/40 (Aβ42/40), phosphorylated-tau 181 (P-tau181), phosphorylated-tau 217 (P-tau217) and neurofilament light (NfL) in non-demented individuals who underwent longitudinal amyloid and tau positron emission tomography (PET), magnetic resonance imaging (MRI) and cognitive testing.
Methods
Blood was collected at baseline from all participants to determine the levels of Aβ42/40, P-tau181, P-tau217 and NfL. All subjects underwent longitudinal 18F-RO948 PET, structural MRI and cognitive assessment. In addition, a subsample also had longitudinal 18F-flutemetamol PET scans. Linear mixed effects models and voxel-wise analyses were applied to assess the relationship between plasma biomarkers and longitudinal changes in imaging and cognition.
Results
Our results show that plasma P-tau217 predicts increases in amyloid (p=0.017) and tau (p=0.004) PET, medial temporal atrophy (p<0.001) and global cognitive decline (p=0.005). The results for the other plasma markers were more variable, with plasma Aβ42/40 predicting amyloid (p=0.002) and tau (p=0.003) PET signal increases as well as cognition (p=0.020), whereas P-tau181 predicts hippocampal atrophy (p=0.008) and cognitive decline (p=0.003), and finally NfL predicts atrophy (p=0.001) and tau PET increases (p=0.020). The comparison between the models showed that the ones that included P-tau217 as a predictor had a significantly better fit to the data as indicated by lower Bayesian Information Criterion values.
Conclusions
These findings suggest that plasma P-tau217 could be useful in clinical trials to determine whether an individual is on the pathophysiological pathway of AD.
ALTERED CEREBRAL BLOOD PERFUSION IN ALZHEIMER’S DISEASE SPECTRUM AND ITS ASSOCIATION WITH AMYLOID-BETA AND TAU PATHOLOGY
Abstract
Aims
A large body of research has shown cerebral blood hypoperfusion across the Alzheimer’s disease (AD) continuum. However, the relationship between the primary AD pathologies and cerebral blood flow (CBF) remains unclear. Here, we examined the link between amyloid-beta (Aβ) and tau with CBF.
Methods
Baseline CBF was measured by arterial spin labeling at a 3T MRI scanner in 94 Aβ- cognitively unimpaired, 43 Aβ+ cognitively unimpaired, and 119 Aβ+ cognitively impaired participants i.e. those with mild cognitive impairment (MCI) or AD dementia. Aβ and tau burden was measured using [18F] flutemetamol and [18F] RO948 positron emission tomography (PET), respectively. Additionally, cerebrospinal fluid (CSF) was analyzed for Aβ42 and Aβ40. Voxel-wise and linear regression analyses were used to assess the interrelation between CBF with Aβ and tau load.
Results
CBF was not associated with amyloid PET or CSF Aβ42/40 in cognitively unimpaired individuals. However, tau PET was inversely related to CBF in lateral temporal, parietal and superior occipital cortices in individuals on the AD continuum i.e. in Aβ+ individuals. Event-based modeling suggested that the observed CBF alterations occurred after neocortical Aβ pathology, temporal tau pathology, and memory deficits, but before widespread neocortical tau pathology.
Conclusions
These findings provide in vivo evidence for an association between tau aggregation and CBF reduction in the AD spectrum and indicate that CBF changes after the occurrence of tau pathology in temporal areas.
PLASMA BIOMARKERS OF ALZHEIMER’S DISEASE PREDICT FUTURE COGNITIVE STATUS IN THE COGNITIVELY UNIMPAIRED ELDERLY
Abstract
Aims
Plasma biomarkers of amyloid-β (Aβ), tau, and neurodegeneration can accurately predict the risk of developing Alzheimer’s disease (AD) dementia in individuals with mild cognitive impairment (MCI), but their effectiveness in the cognitively unimpaired (CU) elderly population is unknown.
Methods
A total of 435 CU individuals were analyzed from the Swedish BioFINDER study with an average age of 72.5 years (range = [60, 88]). We tested the combined ability of plasma Aβ42/Aβ40, tau phosphorylated at threonine 217 (P-tau217), and neurofilament light (NfL) to predict (a) continuous four-year decline in Mini Mental State Examination (MMSE) scores, (b) which individuals remained cognitively stable (< 2 points decline). Plasma biomarkers were compared to a basic model which included only age, sex, and education.
Results
Adding plasma biomarkers to a basic demographics model significantly improved the prediction of continuous four-year MMSE decline (P=0.001) with P-tau217 and NfL having a significant effect (P=0.013 and 0.008, respectively). Adding plasma biomarkers also significantly improved prediction of cognitively stable individuals (P=0.0004), with P-tau217 and NfL again both having a significant effect (P=0.015 and 0.036, respectively). Figure 1 shows the relationship between biomarker levels and four-year change in MMSE (shown as baseline MMSE - four-year MMSE; positive values indicate worsening cognition).
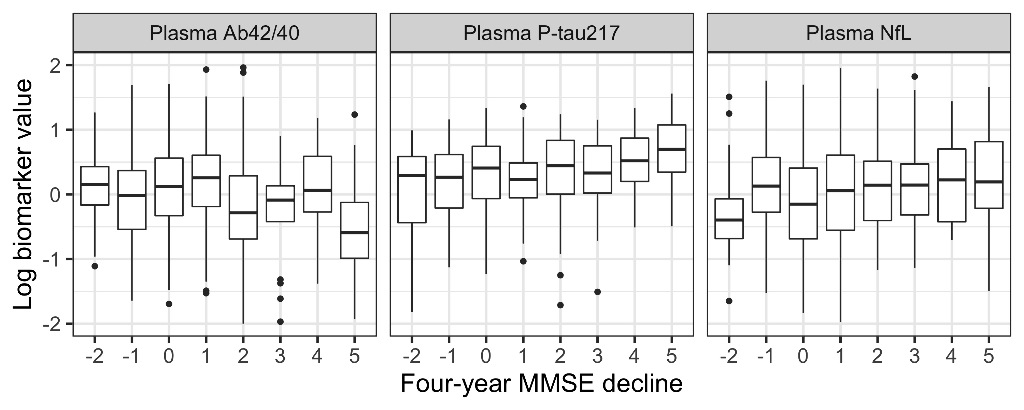
Conclusions
Plasma biomarkers of AD add significant prognostic information for the future cognitive status of elderly individuals without cognitive impairment, particularly for individuals at highest risk of cognitive decline. This further motivates their use in a screening context.
LIVE DISCUSSION
PLASMA AMYLOID, P-TAU217, NFL, AND GFAP AS BIOMARKERS OF AMYLOID PATHOLOGY IN COGNITIVELY HEALTHY INDIVIDUALS
Abstract
Aims
To study if the accuracy of blood amyloid-β (Aβ) to detect Alzheimer disease (AD) at early stages could be improved by other blood biomarkers including phospho-tau217 (P-tau217), neurofilament light (NfL) and glial fibrillary acidic protein (GFAP).
Methods
We measured plasma Aβ42/Aβ40 (Araclon mass spectrometry [MS] or Euroimmun ELISA [EL]), Ptau-217 (Lilly assay), NfL (Simoa assay) and GFAP (Simoa assay) in cognitively unimpaired elderly from the Swedish BioFINDER-1 (n=242) and BioFINDER-2 (n=338) studies. In BioFINDER-1, out of 239 individuals followed longitudinally, 12 converted to AD dementia. CSF Aβ42/Aβ40 measures were available in all study participants, 568 individuals underwent Aβ-PET.
Results
In BioFINDER-1, plasma Aβ42/Aβ40MS identified individuals with abnormal CSF Aβ status with AUC of 0.79 (AIC=260). We observed a better performance (higher AUC and lower AIC) for models including plasma Aβ42/Aβ40MS and P-tau217 (AUC=0.83, AIC=230) or plasma Aβ42/Aβ40MS, P-tau217 and GFAP (AUC=0.85, AIC=224). In BioFINDER-2, the model with plasma Aβ42/Aβ40EL and P-tau217 as predictors provided better fit (AUC=0.84, AIC=319) for CSF Aβ status than plasma Aβ42/Aβ40EL alone (AUC=0.74, AIC=354). The results when using Aβ-PET instead of CSF Aβ status were very similar. In BioFINDER-1, a combination of plasma Aβ42/Aβ40MS and P-tau217 (AUC=0.82, AIC=88) better modelled conversion to AD dementia than plasma Aβ42/Aβ40MS (AUC=0.79, AIC=90). There was no further improvement when adding plasma NfL to the models.
Conclusions
The accuracy of blood test to detect preclinical cerebral Aβ pathology could be improved by combining plasma measurements of Aβ42/Aβ40, Ptau-217 and potentially GFAP.
Presenter of 2 Presentations
PREDICTION OF ALZHEIMER’S DISEASE DEMENTIA USING PLASMA P-TAU217 IN COMBINATION WITH OTHER NON-INVASIVE MEASURES: LONGITUDINAL STUDY FROM THE BIOFINDER AND ADNI COHORTS
Abstract
Aims
To examine the accuracy of plasma phospho-tau217 (P-tau217) for predicting Alzheimer’s disease dementia (AD) when combined with other non-invasive biomarkers. The accuracy was compared to the clinical diagnostic prediction.
Methods
328 participants with subjective cognitive decline (SCD, n=155) or mild cognitive impairment (MCI, n=173) at baseline were included from the Swedish BioFINDER study. Plasma P-tau217, cognitive tests, demographics, plasma Aβ42/40, plasma neurofilament light (NfL), and MRI (AD-signature cortical thickness) were examined using logistic regression models with conversion to AD at 2, 4 and 6 years, respectively, as outcomes. Model selection and area under the ROC curve (AUC) were validated in 531 participants with SCD or MCI from the ADNI study using plasma P-tau181 instead of P-tau217.
Results
Plasma P-tau217 predicted conversion to AD with an AUC of 0.78 (95%CI 0.72-0.85) at 2 years, 0.82 (0.77-0.88) at 4 years and 0.85 (0.80-0.90) at 6 years. 2-year prediction of AD was improved by adding MRI, memory, executive function, and gender to P-tau217 (0.88, 0.83-0.94). 4-year prediction was improved by adding MRI, memory, and executive function (0.90, 0.86-0.93). 6-year prediction was improved by adding plasma Aβ42/40 and NfL, MRI, and memory (0.91, 0.87-0.94). In ADNI, similar models were selected with similar accuracies. The diagnostic prediction of memory clinic physicians blinded to biomarker data were inferior to all models (AUCs 0.68-0.73).
Conclusions
Plasma P-tau can accurately predict future AD, better than a clinical diagnostic prediction. The accuracy can be improved further by adding brief cognitive tests and MRI, and to a lesser extent plasma Aβ42/40 and NfL.



