PERFORMANCE OF THE LILLY AUTOMATED INSULIN DELIVERY (AID) SYSTEM: RESULTS OF EARLY PHASE FEASIBILITY STUDY
- Amy K. Bartee, United States of America
- Amy K. Bartee, United States of America
- Amy Lalonde, United States of America
- Michelle Katz, United States of America
- Ronald Brazg, United States of America
- Mark Christiansen, United States of America
- Howard Wolpert, United States of America
- Richard Jones, United States of America
Abstract
Background and Aims
AID systems have led to demonstrated improvements in time in range without increasing hypoglycemia. Further advances in performance and usability will set the stage for more widespread adoption of this technology in diabetes self-management.
Methods
This inpatient early feasibility study evaluated the Lilly hybrid closed loop system comprising a high accuracy pump (developed by DEKA Research & Development Corp.), Lispro insulin, pump-embedded model predictive control algorithm, Dexcom continuous glucose monitor, and smartphone-based controller. Subjects underwent two similar 2-day inpatient protocols (10 adults with type 1 diabetes per protocol) including meal-related challenges (~30% over-bolus and 1-hour delayed bolus encompassing carbohydrate coverage and hyperglycemia correction) to simulate real-world diabetes self-management errors.
Results
Subjects (25% male) had mean age: 44.7±14.2 years, T1D duration: 30.2±11.1 years, A1C: 7.2±0.8%, and insulin usage: 0.53±0.21 U/kg/day. The table below describes glucose control (percent time in different ranges [mean±SD]) while the subjects were on the AID system.
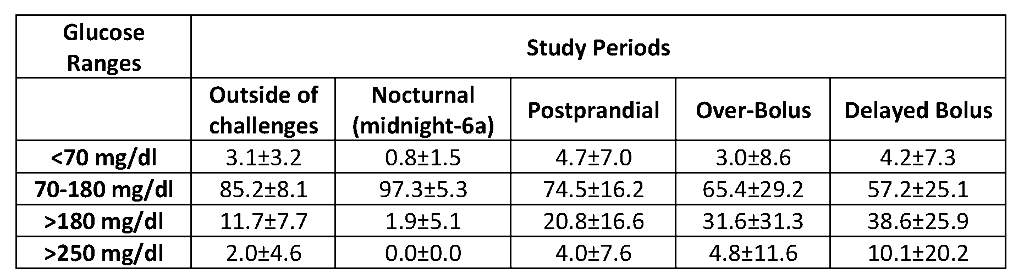
Conclusions
In this closely supervised feasibility study the Lilly AID system demonstrated expected algorithm performance in response to simulated diabetes self-management errors and promising glycemic outcomes. Clinical trial evidence in more ‘real world’ environments will provide additional information on the safety and effectiveness of the system.