Welcome to the AD/PD™ 2021 Interactive Program
The congress will officially run on Central European Time (CET) - Barcelona Time
To convert the congress times to your local time Click Here
Icons Legend: ![]()
 - Live Session |
- Live Session |  - On Demand Session |
- On Demand Session |  - On Demand with Live Q&A
- On Demand with Live Q&A
The viewing of sessions, cannot be accessed from this conference calendar. All sessions are accessible via the Main Lobby.
FOLLOWING THE LIVE DISCUSSION, THE RECORDING WILL BE AVAILABLE IN THE ON-DEMAND SECTION OF THE AUDITORIUM.
ALZHEIMER THERAPEUTICS: POTENTIAL PIPELINE PREDICTIONS
Abstract
Aims
Alzheimer’s disease (AD) has a complex biology with many potential processes that might comprise targets for therapy.
Methods
We reviewed clinicaltrials.gov the US Food and Drug Administration (FDA) database to determine the number of agents in all phases of the AD drug development pipeline as reflected in this registry. We use the NIH Common AD Research Ontology (CADRO) categories to determine the targets and therapeutic categories of the agents in trials.
Results
There are currently (of as of 2/27/2020) 121 unique agents in trials; 29 in Phase 3, 65 in Phase 2, and 27 in Phase 3. Amyloid-beta protein and hyperphosphorylated tau protein are important therapeutic targets --- 16 and 11 agents in the pipeline respectively --- and account for a major portion of the drugs in the AD pipeline. Neuroinflammation/inflection (20 agents) and synaptic plasticity/neuroprotection (20 agents) have the largest number of agents in trials across Phase 1, Phase 2, and Phase 3 trials. Metabolic/bioenergetic agents are being assessed in Phase 2 (6 agents) and Phase 1 (3 agents) trials. Eighty percent of the agents in the pipeline (97 of 121) are putative disease modifying drugs; 10% target cognitive enhancement and 10% address neuropsychiatric manifestations of AD. Of the total number of trials for drugs in the pipeline, 6 are in preclinical AD, 39 are in prodromal AD, and 45 are in AD dementia.
Conclusions
The AD drug development pipeline is beginning to deliver drugs that address important unmet needs of patients with AD or those at risk for the disease.
CREATING PATHWAYS FOR BREAKTHROUGHS IN ALZHEIMER’S DISEASE
Abstract
Abstract Body
Creating Pathways for Breakthroughs in Alzheimer’s Disease
Stephen Salloway, M.D., M.S.
Martin M. Zucker Professor of Neurology and Psychiatry
Warren Alpert Medical School of Brown University
Alzheimer’s disease represents an epidemic within a pandemic in our aging world. The disease currently has no meaningful treatment to slow progression but we are on the verge of breakthroughs in Alzheimer’s disease research thanks to the inspiring contributions of thousands of study participants and the dedication of talented researchers. This presentation will review the progress we have made and the challenges we face to create pathways for major advances in diagnosis and treatment.
In 2012 the United States launched the National Plan to Address Against Alzheimer’s Disease which has led to a dramatic increase in AD research funding from the National Institutes of Health. The NIH has partnered with industry and foundation funders to create landmark observational trials that have advanced our understanding of the evolution of AD pathogenesis. Advances in molecular brain imaging can now safely detect AD pathological changes years before cognitive decline, opening the era for Alzheimer’s prevention. Drugs that modify amyloid and tau to slow the disease course are currently under regulatory review. Promising plasma and digital biomarkers will soon be available to make early diagnosis and screening for trials and treatment more widely available and cost-effective. Encouraging gene-modifying strategies that have shown important benefits in other neurological diseases are now being tested for AD and other neurodegenerative disorders.
Many important challenges lay ahead that include expanding public-private partnerships to meet our research goals and prioritizing the training of talented young investigators to develop the necessary research work force, We will need to implement practical screening tests and access to emerging treatments on a global scale and multi-targeted discovery science and lifestyle interventions to pioneer combination treatments.
RATIONALE FOR THE PHASE II CLINICAL TRIAL ASSESSING THE SAFETY, TOLERABILITY, AND EFFICACY OF LENALIDOMIDE IN MILD COGNITIVE IMPAIRMENT DUE TO ALZHEIMER’S DISEASE
Abstract
Aims
Accumulating evidence indicates that NF-kappa B, TNFα, interleukins (IL-1beta, IL-2, and IL-6), and chemokines (IL-8) are elevated in the blood and central nervous system of AD patients suggestng that inflammation plays a central role in the cause and effect of AD neuropathology. The immunomodulator, anti-cancer agent lenalidomide is a pleiotropic agent that both lowers the expression of TNFα, IL-6, IL-8, and increases the expression of anti-inflammatory cytokines (e.g. IL-10), to modulate both innate and adaptive immune responses. We aim to test the central hypothesis that lenalidomide reduces inflammatory and AD-associated pathological biomarkers, and improves cognition.
Methods
For this, we designed an 18-month, Phase II, double-blind, randomized, placebo controlled, and proof-of-mechanism clinical study in amnestic mild cognitive impairment due to AD; aMCI). The effects of lenalidomide treatment will be assessed after 12 months of treatment and 6 months washout (month 18). The primary aim: to assess the effect of lenalidomide on cognition after 12 months of treatment. The secondary aim is to assess the safety and tolerability of lenalidomide in aMCI patients. The tertiary and exploratory aims: We will investigate the potential of blood inflammatory markers as surrogate markers of the therapeutic efficacy of the study drug.Lenalidomide 10 mg/day vs. placebo taken daily orally (ratio 1:1) in 30 subjects with aMCI due to AD. Age range 50-90 with standard I/E criteria
Results
baseline data and rationale will be presented
Conclusions
Our study should determine whether lenalidomide is safe in AD subjects and whether it can alter the clinical progression of AD when administered before dementia onset
NEUROPSYCHOLOGICAL AND NEUROPSYCHIATRIC ASSESSMENTS IN ALZHEIMER’S DISEASE PATIENTS TREATED WITH PLASMA EXCHANGE WITH ALBUMIN REPLACEMENT FROM THE AMBAR STUDY
Abstract
Aims
Recently, the primary results of cognitive, functional, and global assessment outcomes of the Alzheimer’s Management By Albumin Replacement (AMBAR) trial demonstrated that plasma exchange (PE) with albumin replacement slowed the cognition and functional decline associated with the progression of the disease. Here we report the AMBAR results on neuropsychological and neuropsychiatric outcomes.
Methods
Out of 496 patients enrolled, 347 were randomized into three PE-treatment arms with different doses of albumin and intravenous immunoglobulin (IVIG) replacement, and placebo (sham PE). Initially, a weekly conventional therapeutic PE (TPE), the same for the three PE-treatment arms, was done for 6 weeks. After this period, each arm underwent a 12-month period of monthly low-volume PE (LVPE). Specific test measurements were performed at month 0 (baseline), month 2 (end of TPE period), months 6, 9 and 12 (during LVPE period), and month 14.
Results
While neuropsychological scores of placebo patients declined across the study, all-patient and mild-AD (MMSE: 22-26) cohorts treated with PE and high-dose albumin plus IVIG showed improvement in language fluency (effect sizes: 106%-362%; p-values: 0.03 to 0.001), and processing speed (effect sizes: 318%-743%; p-values: 0.03 to 0.04) at month 14. All-patient and moderate-AD (MMSE: 18-21) cohorts significantly improved short-term verbal memory (effect sizes: 94%-1100%; p-values: 0.02 to 0.003). The progression of the neuropsychiatric symptoms, including depression and suicide, of PE-treated and placebo patients was similar to placebo across the study.
Conclusions
PE-treated AD patients showed improvement in specific neuropsychological tests of memory and language, without a worsening of their psychoaffective status.
ADCOMS: A POST-HOC ANALYSIS USING DATA FROM THE 3-YEAR LIPIDIDIET TRIAL IN PRODROMAL ALZHEIMER’S DISEASE
Abstract
Aims
Objectives: LipiDiDiet1 is a 6-year, double-blind, parallel-group, multi-center, randomized controlled clinical trial, investigating the specific multinutrient combination Fortasyn Connect in prodromal AD. The main aim of the present post-hoc analysis was to explore the effects of a 3-year multinutrient intervention on cognition and global function, and its subdomains, as captured by ADCOMS, a composite shown to be sensitive to change in individuals with prodromal AD, using data from the LipiDiDiet trial.
Methods
Methods: Of the study population of 311, 138 active and 140 control participants were applicable for ADCOMS analysis. ADCOMS was calculated using the selected items and corresponding partial least squares coefficients and the change from baseline was the outcome. LS-means over 36 months were calculated using a linear mixed model for longitudinal data that included baseline as a covariate, continuous time, and site and subject ID as a random effect.
Results
Results: A significant benefit of the active intervention was observed using the ADCOMS score over 36 months (P=0.045; 36% slowing). Changes were primarily driven by the CDR-SB (P=0.024; 36% slowing). In addition, ADAS-cog and MMSE showed trends consistent with improvement (P>0.05; 7 and 70% slowing, respectively). Sensitivity models showed similar results.
Conclusions
Conclusions: In this post-hoc analysis of the LipiDiDiet study data, the active group showed significantly less clinical decline over 36 months as measured by ADCOMS, confirming long-lasting beneficial effects of the multinutrient in a prodromal AD population. These analyses further validate ADCOMS in early AD.
1EUFP7 N°211696 LipiDiDiet
LM11A-31 SMALL MOLECULE P75 RECEPTOR MODULATOR –PRECLINICAL ASSESSMENT AND BIOMARKERS RELEVANT TO A PHASE 2A EXPLORATORY TRIAL IN ALZHEIMER’S DISEASE
Abstract
Aims
In the context of outcome measures in APP- and tauopathy-based mouse models, assess exploratory endpoint measures to be applied in a phase 2a trial in mild-moderate Alzheimer’s disease (AD) for LM11A-31, a first-in-class small molecule modulating p75 neurotrophin receptor signaling.
Methods
Studies incorporating APP-L/S, Tg-2576 and P301S (PS19) mice included oral gavage for three-month treatment periods commencing after establishment of amyloid- and tau-related pathology. Outcome measures included p75 receptor-linked downstream signaling; accumulation of pathological tau species and tau seeding activity; neurite, synaptic and spine degeneration; measures of microglia and astrocyte status; and behavioral assessment. A phase 2a exploratory endpoint trial in mild-moderate AD contains three study arms: placebo, low-dose and high-dose administered by oral capsules for 6 months. Baseline and post-treatment assessments include: ADAS-Cog-13, a Neurological Testing Battery and other cognitive tests; CSF biomarkers; volumetric MRI and statistical region of interest FDG-PET.
Results
Mouse model studies suggest relevant biomarkers available in the context of a clinical trial. A total of 242 from a target 240 subjects were enrolled. Randomization resulted in similar populations across the three study arms. Within-subject baseline and post-treatment FDG-PET imaging, MRI imaging and CSF was obtained from approximately 65%. Data assessment is underway.
Conclusions
Consideration of preclinical studies guides the application of imaging and CSF biomarkers in this exploratory endpoint trial. The treatment phase of the 2a trial has been completed. Formal analyses following data lock will assess primary safety as well as exploratory imaging, CSF biomarker and cognitive endpoints.
ACCOUNTING FOR INTERVAL-CENSORING IN LONGITUDINAL ALZHEIMER'S DATASETS IMPROVES SURVIVAL MODELLING ESTIMATES
Abstract
Aims
Many longitudinal Alzheimer's disease (AD) studies collect measurements every 12 or 18 months, creating uncertainty as to when discrete events (change in clinical diagnosis, conversion from amyloid negative to positive) occur. However, most time-to-event analyses assume the time an event occurs is known. We examine whether accounting for uncertainty associated with infrequent observations impacts common survival estimates.
Methods
We contrast two Weibull survival models in a Bayesian framework, denoted ‘standard’ and ‘interval-censored’, which assume the event occurs 1) exactly at the most recent visit and 2) anytime between the most and second-most recent visits. Both models, implemented in Stan, are parametrized by shape (μ), scale (α) and model coefficients (β)
We simulate conversion from amyloid negative to positive for 500 individuals, with measures of PET Amyloid, APOE4, sex and age. The distributions for events and measures are based on those observed in the Australian Imaging, Biomarkers and Lifestyle (AIBL) study for people with at least two complete data points. 14% of individuals converted during the observation period.
Results
On simulated data, the interval-censored model more accurately estimates baseline survival distribution and adjustment coefficients than the standard approach (Figure 1a). Furthermore, the Bayesian interval-censored model allows us to examine the posterior distributions of `true' event times to gain individual-level estimates as to when the event may have occurred in the censored interval (Figure 1b).
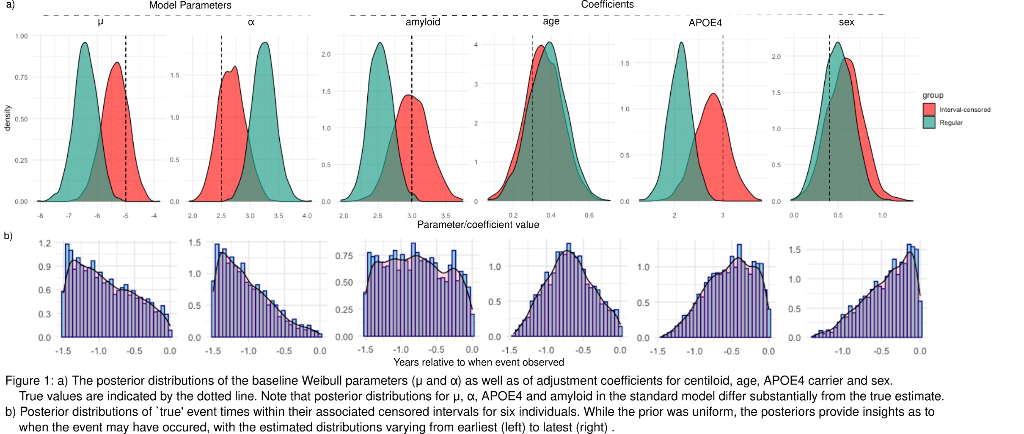
Conclusions
Accounting for interval censoring can strongly impact key survival estimates and may alter model interpretations. We are extending this to other (semi-)parametric models (e.g. Cox regression) and exploring its impact on real-world data.


