Welcome to the AD/PD™ 2021 Interactive Program
The congress will officially run on Central European Time (CET) - Barcelona Time
To convert the congress times to your local time Click Here
Icons Legend: ![]()
 - Live Session |
- Live Session |  - On Demand Session |
- On Demand Session |  - On Demand with Live Q&A
- On Demand with Live Q&A
The viewing of sessions, cannot be accessed from this conference calendar. All sessions are accessible via the Main Lobby.
FOLLOWING THE LIVE DISCUSSION, THE RECORDING WILL BE AVAILABLE IN THE ON-DEMAND SECTION OF THE AUDITORIUM.
DIFFERENTIATION AND CHARACTERIZATION OF CEREBRAL ORGANOIDS FROM HUMAN IPS CELLS WITH A PSEN1 MUTATION VS. ISOGENIC CONTROLS
Abstract
Aims
Within this study we aimed to develop a new cerebral organoid model to study mechanisms underlying familial Alzheimer’s disease. Human induced pluripotent stem cells (hiPSC) with a PSEN1 mutation and the isogenic control cells should be analysed in respect to their potential to differentiate cerebral organoids.
Methods
The methods included stem cell culture, differentiation of embryonic bodies and cerebral organoids, the freezing, slicing, and the staining of the organoids. Differentiation of cerebral organoids was assessed using primary antibodies against PAX6, SOX2, OCT4, MAP2, betaTubulinIII, and FOXG1. Antibodies against human beta amyloid, phosphorylated Tau, and AICD were used to identify Alzheimer disease-relevant changes in the PSEN+/+, PSEN+/-, and PSEN-/- (wt) organoid slices. The stained and mounted samples were imaged with the Leica TCS SP8 confocal microscope.
Results
The PSEN1 cerebral organoids revealed normal differentiation including development of PAX6+ precursor cells and mature bTubIII+ neurons, which together established a cortical plate-like brain structure. Immunofluorescence analysis of sliced organoids revealed cell-type specific APP processing and expression of PSEN1.
Conclusions
Due to the organization of PSEN1 cerebral organoids mimicking the human brain and due to their human origin, they correspond to a promising model system to study Alzheimer’s disease relevant pathways, which are of relevance for familial as well as for sporadic AD. Robust expression of APP and PSEN1 also empower the model system for drug treatment screens and identification of reliable biomarkers.
NEURONAL TAU PATHOLOGY TRANSFERS TO ASTROCYTES AND INDUCES THEIR LOSS ACCORDING TO TAU AGGREGATION IN MOUSE MODELS
Abstract
Aims
Tauopathies are neurodegenerative disorders characterized by the accumulation of aggregated Tau in brain cells and neuronal loss. For decades, efforts have essentially focused on neuronal pathology whereas several Tauopathies also display glial Tau pathology. Hence, the mechanisms leading to astrocytic Tau lesions have been little explored. Here we investigated the origin of astrocytic Tau, and the consequences of Tau pathology on astrocyte morphology and survival.
Methods
We modelled Tau pathology using AAV gene transfer to overexpress Tau in the hippocampus of adult C57Bl/6J mice. Tau pathology was studied by immunohistology, in situ hybridization and confocal microscopy. Stereological counting of Sox-2 positive astrocytes assessed astrocytes survival. To describe astrocytes’ morphology, we injected intravenously 8-months old Thy-Tau22 mice with a PHP.eB AAV expressing tdTomato under an astrocyte-specific promoter and used confocal microscopy combined with 3-dimensional reconstruction.
Results
Astrocytic Tau lesions appeared secondarily to neuron- induced Tau pathology in both our AAV-based models and in aged Thy-Tau22 male mice. We further evidenced that Tau species do transmit from neurons to astrocytes. Astrocyte numbers were significantly reduced in the presence of soluble hyperphosphorylated Tau pathology, especially in the subiculum, whereas they remained stable in the presence of mature Tau lesions up to 3 months after AAV injection. The study of astrocytes' morphology in aged Thy-Tau22 mice is undergoing.
Conclusions
Our data show that astrocytes are not mere by-standers of neuronal pathology. Consistent with what we previously reported for neurons, we revealed major astrocytic vulnerability in the presence of soluble hyperphosphorylated Tau pathology.
SPATIOTEMPORAL ALTERATIONS OF RESTING-STATE QUASI-PERIODIC PATTERNS IN 4-MONTH OLD TGF344-AD RATS
Abstract
Aims
Alzheimer’s Disease (AD) is a severe neurodegenerative disorder that leads to brain network dysfunction and cognitive decline. Changes in functional networks at symptomatic stages of AD can be captured using resting state (RS) fMRI in patients and animal models. Here, we used a rat model manifesting the full-spectrum of human AD-pathology to identify if spatiotemporal network alterations are present at an early, pre-symptomatic stage.
Methods
RS fMRI data were collected from four-month-old TgF344-AD rats (N=15) and wildtype (WT) (N=11) littermates. Acquired images were realigned, normalized to a 3D- template, masked, smoothed, filtered and the global signal was regressed out. Recurrent patterns of brain activity (3.6 seconds long) were extracted using quasi-periodic pattern (QPP) analysis starting from 200 different seed patterns. Then, the 200 patterns of each group were clustered based on temporal and spatial similarity to identify the representative QPPs based on occurrence rates.
Results
Voxel-wise activations of matched QPPs across groups (Fig 1A)were compared using a two-sample t-test, FDR corrected for multiple comparisons. Significant QPP differences between groups were primarily found in the basal forebrain (BFB), and cingulate cortex (Cg) (Fig 1B). QPP time-course analysis in these regions-of-interest and somatosensory cortex (SS), demonstrated a concomitant reduction of BFB and an increase of Cg activity in the Tg-rats compared to the WT controls, while SS activity profiles remained unchanged (Fig 1C).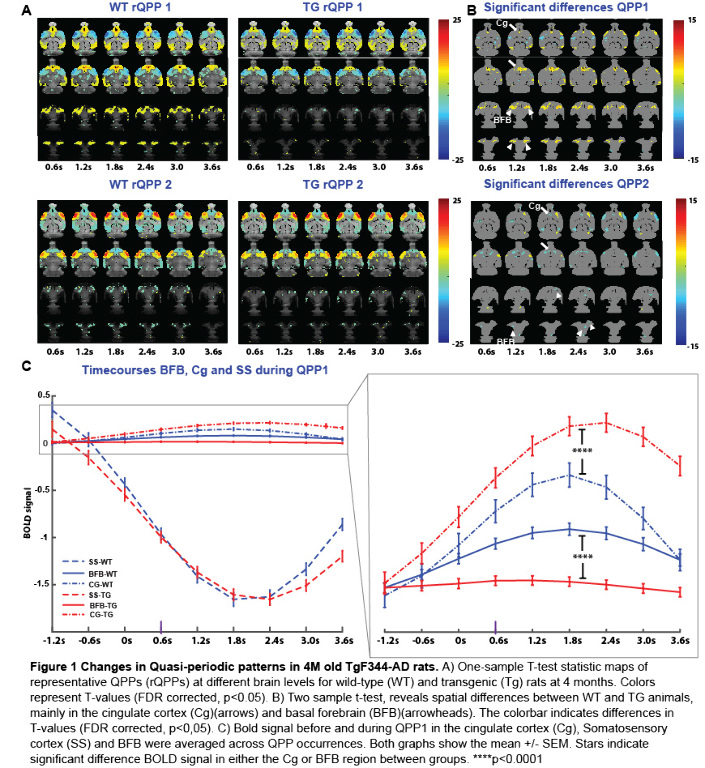
Conclusions
In summary, our results highlight the important role of the BFB in regulating whole-brain networks and indicate a potential signature to identify early onset changes at the network level.
PULSE-CHASE PROTEOMICS OF APP KNOCK-IN MOUSE MODELS OF AMYLOID PATHOLOGY REVEALS SYNAPTIC DYSFUNCTION ORIGINATES IN PRESYNAPTIC TERMINALS
Abstract
Aims
Alzheimer’s disease (AD) is pathologically characterized by the presence of protein deposits in amyloid plaques and neurofibrillary tangles. However, an understanding of the mechanisms responsible for impaired protein degradation in AD is lacking. Our goal is to identify proteins with impaired turnover during the early stage of Aβ42 accumulation in vivo and determine how this process may contribute to AD etiology.
Methods
We performed 15N stable isotope metabolic pulse-chase labeling with liquid chromatography tandem-mass spectrometry (MS)- based proteomic analysis of App knock in mice. We monitored protein turnover in multiple brain regions across different stages of Aβ proteotoxicity in AppNL/NL, AppNL-F/NL-F, AppNL-G-F/NL-G-F models at six and 12 months of age. We also used immunohistochemistry, thioflavin, immunohistochemistry, electron microscopy, electrophysiology, and multi-isotope imaging MS, full methods can be found our upcoming Cell Systems publication.
Results
We discovered that the axon terminal represents the predominant cellular compartment with hampered proteostasis, as presynaptic proteins have impaired turnover just as Aβ42 accumulation becomes detectable. These proteins have elevated levels - not due to compensatory increases in mRNA abundance. Multiple synaptic vesicle (SV) associated proteins accumulated in both amyloid plaque-dependent and -independent manners. Aβ and APP interact with SV proteins, and Aβ42 impedes SV fusion. The SV pool is enlarged, and short-term potentiation is elevated before hippocampal synapse density is reduced or synaptic transmission becomes impaired.
Conclusions
Axon terminals are among the earliest and most vulnerable cellular compartments affected by AD-like pathology and may play a pioneering role in the establishment of synaptotoxicity and cognitive impairment in AD.
DYSREGULATION OF WNT SIGNALLING IN APP KNOCK-IN ALZHEIMER'S DISEASE MODELS
Abstract
Aims
Dysfunction of Wnt signalling has been shown to play an important role in synaptic dysfunction and amyloidosis, contributing to the development of Alzheimer's disease (AD). Although studies have shown alterations of Wnt signalling components in transgenic (Tg) mouse models overexpressing mutant amyloid-β precursor protein (APP), more detailed investigations of underlying molecular signalling pathway dysfunction are required in AD models representing AD progression in human patients more accurately. In this study, the effect of pathogenic APP mutations on Wnt signalling was investigated using App knock-in AD mouse models (App NL-F).
Methods
Wnt signalling activity was detected by TCF/LEF luciferase reporter assays in primary neuronal cultures. mRNA and protein levels of Wnt signalling in mice of different ages were investigated by qPCR and Western Blot respectively. Protein expressions and distributions were further examined by immunohistochemistry.
Results
Results showed that Wnt signalling activity was decreased in primary AppNL-F/NL-F cortical and hippocampal cultures in comparison to wild-type cultures. Levels of β-catenin, the key Wnt signalling mediator, decreased significantly in App knock-in mice compared to wild-type mice. Protein levels of Wnt ligands, other Wnt signalling components and target genes, including Wnt5a, LRP6, Axin2, CyclinD1 and BDNF also showed significant reductions in App knock-in mice compared to wild-type mice.
Conclusions
In conclusion, App knock-in mice showed a reduction in Wnt signalling activity, indicating a down-regulation of this signalling pathway in the early stages of AD pathogenesis. Future work will focus on the role of Wnt signalling on morphological changes in primary neurons.
PERSISTENT RELIEF OF MOTOR SYMPTOMS IN A PARKINSONIAN MOUSE MODEL AFTER OPTOGENETIC STIMULATION OF MOTOR CORTEX AND D2 RECEPTOR ACTIVATION
Abstract
Aims
Motor deficits in Parkinson’s disease (PD) results from dopamine loss. Our earlier studies suggest an important role of task-specific aberrant inhibitory learning mediated by long-term potentiation in corticostriatal synapses onto D2 receptor expressing striatal neurons (D2-MSNs) in the absence of dopamine. We hypothesize that corticostriatal stimulation along with D2 receptor activation would reverse such aberrant plasticity and reduce motor deficits in PD.
Methods
Motor deficits of unilateral 6-OHDA lesioned mice were assessed using rotarod and rotation tests. Postmortem tyrosine hydroxylase (TH) immunostaining confirmed dopamine loss. AAV2 containing channelrhodopsin-2 was expressed in layer 5 motor cortex projection neurons. Optogenetic high-frequency stimulation (oHFS) of corticostriatal projections was combined with D2 agonist quinpirole to induce long term depression (LTD) of D2-MSN inputs. Motor performance was monitored using rotarod. Electrophysiological studies were conducted ex-vivo to assess corticostriatal plasticity and excitability of D2-MSNs in sham and lesioned animals.
Results
6-OHDA animals displaying strong motor deficits received oHFS in the dorsolateral striatum (DLS) along with quinpirole administration for 5 days. This treatment improved motor performance for over 4 weeks. oHFS or quinpirole alone did not improve the motor performance. In ex-vivo electrophysiology, oHFS-induced LTD was obtained using bath application of quinpirole in 6-OHDA mice. Consistent with LTD induction, VGLUT1 expression in DLS was reduced in combined treatment received animals.
Conclusions
Our results indicate that combination of cortical stimulation and D2 receptor activation reverses the aberrant plasticity in DLS, resulting in long-lasting improvement in motor function, suggesting an effective therapeutic approach for PD.
NEURONAL UPREGULATION OF ADENOSINE A2A RECEPTORS EXACERBATES MEMORY DEFICITS IN MOUSE MODELS OF ALZHEIMER'S DISEASE
Abstract
Aims
Alzheimer’s Disease (AD) is characterized by memory loss, underlined by synaptic impairments promoted by both amyloid and Tau lesions. Various studies pointed-out that chronic caffeine consumption reduces Alzheimer's Disease risk and associated cognitive deficits. These protective effects are thought to be ascribed to the blockade of adenosine A2A receptors (A2ARs), known to be crucial for synaptic tuning. Interestingly, A2ARs are found abnormally upregulated in neurons of aged individuals and AD patient’s brains in correlation with the development of cognitive deficits, suggesting a link between neuronal A2AR dysregulation, synapse dysfunction and memory impairments in AD.
Methods
To address the contribution of pathological upsurge of A2AR in AD, we use new transgenic and viral approaches to upregulate neuronal A2AR in the THY-Tau22 model of Tau pathology and the APP/PS1dE9 model of amyloidogenesis. Neuronal upsurge of A2AR was elicited at early pathological stages in both models, at times they do not exhibit memory deficits. These genetic approaches were combined with behavioral, histological, biochemical and molecular (RNA-Seq, mass spectrometry-based high- throughput proteomics) investigations.
Results
Our data revealed that A2AR overexpression worsened AD-related spatial memory impairments without significantly altering Tau and amyloid lesions nor canonical neuroinflammatory markers. Importantly, in both AD models, A2AR-mediated memory impairments were associated with a significant synaptic loss, involving different mechanisms.
Conclusions
These data support that pathological upregulation of A2AR in AD contributes to synaptic and cognitive impairments by differential and convergent pathways paving the way for a therapeutic use of A2AR antagonists.
OSTEOPONTIN MARKS A SUBPOPULATION OF BRAIN MACROPHAGES THAT CONTRIBUTES TO SYNAPSE LOSS IN EARLY ALZHEIMER MOUSE MODELS
Abstract
Aims
Microglia and CNS-resident macrophages exhibit heterogeneous phenotypes dependent on spatiotemporal context, allowing them to fulfill various functions in health and disease. Emerging data suggest that microglia mediate synaptic loss in Alzheimer’s disease (AD), yet we know little about the underlying mechanisms and potential subsets or cell states involved. Here we report SPP1/Osteopontin, a glycoprotein involved in phagocytosis, as a marker of CNS-resident macrophages that mediate synaptic elimination in AD.
Methods
To measure SPP1 levels in brain regions and time points when synapses are vulnerable to loss, we performed in-situ hybridization, flow cytometry and immunohistochemistry in multiple mouse models of AD including APPNL-F knock-in and acute model of Aβ oligomer-induced synapse loss. To confirm cellular source, we performed cell-type specific staining for macrophages in-situ. To assess the role of SPP1 in synapse loss, we performed super-resolution imaging to quantify synaptic puncta in Aβ oligomer- vs control-challenged Spp1-, C1qa- and Itgam- knock-out mice.
Results
We found a brain-region specific upregulation of SPP1 mRNA and protein in particular subset of CNS-resident macrophages, coinciding at a time point when synapses are vulnerable to engulfment. In Spp1-knockout mice, Aβ oligomers were unable to induce synapse loss as compared to wild-type controls. C1qa and Itgam- knock-out mice fail to upregulate SPP1 in response to Aβ oligomers, suggesting that SPP1 upregulation in this macrophage subset is downstream of complement activation.
Conclusions
Our data suggest SPP1 as a mediator of synapse loss in pre-plaque AD mouse models, raising SPP1 as a potential biomarker target of cells aberrantly eliminating synapses.


