
Monica Van den Berg, Belgium
University of Antwerp Bio-imaging labAuthor Of 2 Presentations
LIVE DISCUSSION
- Thorsten Mueller, Germany
- Karine Cambon, France
- Monica Van den Berg, Belgium
- Tiansheng Liu, United Kingdom
- Chandrika Abburi, United States of America
- Jeffrey N. Savas, United States of America
- Rick Livesey, United Kingdom
- David Morgan, United States of America
- David Blum, France
- Sebastiaan De Schepper, United Kingdom
SPATIOTEMPORAL ALTERATIONS OF RESTING-STATE QUASI-PERIODIC PATTERNS IN 4-MONTH OLD TGF344-AD RATS
Abstract
Aims
Alzheimer’s Disease (AD) is a severe neurodegenerative disorder that leads to brain network dysfunction and cognitive decline. Changes in functional networks at symptomatic stages of AD can be captured using resting state (RS) fMRI in patients and animal models. Here, we used a rat model manifesting the full-spectrum of human AD-pathology to identify if spatiotemporal network alterations are present at an early, pre-symptomatic stage.
Methods
RS fMRI data were collected from four-month-old TgF344-AD rats (N=15) and wildtype (WT) (N=11) littermates. Acquired images were realigned, normalized to a 3D- template, masked, smoothed, filtered and the global signal was regressed out. Recurrent patterns of brain activity (3.6 seconds long) were extracted using quasi-periodic pattern (QPP) analysis starting from 200 different seed patterns. Then, the 200 patterns of each group were clustered based on temporal and spatial similarity to identify the representative QPPs based on occurrence rates.
Results
Voxel-wise activations of matched QPPs across groups (Fig 1A)were compared using a two-sample t-test, FDR corrected for multiple comparisons. Significant QPP differences between groups were primarily found in the basal forebrain (BFB), and cingulate cortex (Cg) (Fig 1B). QPP time-course analysis in these regions-of-interest and somatosensory cortex (SS), demonstrated a concomitant reduction of BFB and an increase of Cg activity in the Tg-rats compared to the WT controls, while SS activity profiles remained unchanged (Fig 1C).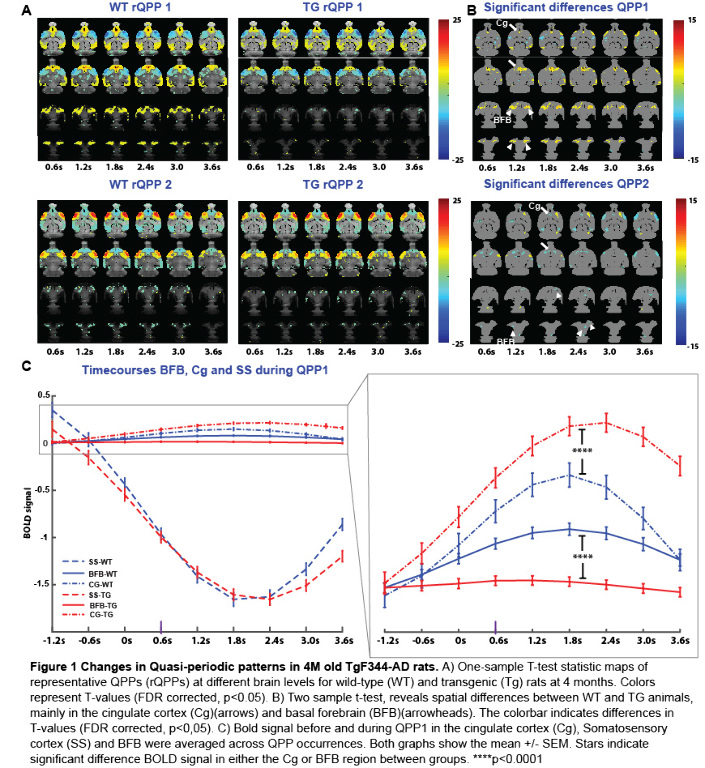
Conclusions
In summary, our results highlight the important role of the BFB in regulating whole-brain networks and indicate a potential signature to identify early onset changes at the network level.
Presenter of 2 Presentations
SPATIOTEMPORAL ALTERATIONS OF RESTING-STATE QUASI-PERIODIC PATTERNS IN 4-MONTH OLD TGF344-AD RATS
Abstract
Aims
Alzheimer’s Disease (AD) is a severe neurodegenerative disorder that leads to brain network dysfunction and cognitive decline. Changes in functional networks at symptomatic stages of AD can be captured using resting state (RS) fMRI in patients and animal models. Here, we used a rat model manifesting the full-spectrum of human AD-pathology to identify if spatiotemporal network alterations are present at an early, pre-symptomatic stage.
Methods
RS fMRI data were collected from four-month-old TgF344-AD rats (N=15) and wildtype (WT) (N=11) littermates. Acquired images were realigned, normalized to a 3D- template, masked, smoothed, filtered and the global signal was regressed out. Recurrent patterns of brain activity (3.6 seconds long) were extracted using quasi-periodic pattern (QPP) analysis starting from 200 different seed patterns. Then, the 200 patterns of each group were clustered based on temporal and spatial similarity to identify the representative QPPs based on occurrence rates.
Results
Voxel-wise activations of matched QPPs across groups (Fig 1A)were compared using a two-sample t-test, FDR corrected for multiple comparisons. Significant QPP differences between groups were primarily found in the basal forebrain (BFB), and cingulate cortex (Cg) (Fig 1B). QPP time-course analysis in these regions-of-interest and somatosensory cortex (SS), demonstrated a concomitant reduction of BFB and an increase of Cg activity in the Tg-rats compared to the WT controls, while SS activity profiles remained unchanged (Fig 1C).
Conclusions
In summary, our results highlight the important role of the BFB in regulating whole-brain networks and indicate a potential signature to identify early onset changes at the network level.
LIVE DISCUSSION
- Thorsten Mueller, Germany
- Karine Cambon, France
- Monica Van den Berg, Belgium
- Tiansheng Liu, United Kingdom
- Chandrika Abburi, United States of America
- Jeffrey N. Savas, United States of America
- Rick Livesey, United Kingdom
- David Morgan, United States of America
- David Blum, France
- Sebastiaan De Schepper, United Kingdom



