- A. Anz (Gulf Breeze, US)
- L. Fortier (Ithaca, US)
18.3.1 - Development of a new technique for directing stem cells to engraft into articular cartilage lesions
- A. Salerno (Liverpool, GB)
- A. Salerno (Liverpool, GB)
- A. Dabbadie (Liverpool, GB)
- L. Lian (Liverpool, GB)
- S. Prendergast (Liverpool, GB)
- B. Poulet (Liverpool, GB)
- A. Hollander (Liverpool, GB)
Abstract
Purpose
Injection of undifferentiated mesenchymal stromal/stem cells (MSCs) into osteoarthritic (OA) joints can lead to a reduction in symptoms (1,2). Inducing the engraftment of MSCs into cartilage lesions may improve their efficacy after injection. We therefore propose to target MSCs through attachment to Type II collagen gelatin. This will be achieved by coating the surface of MSCs with a mutated module of the collagen binding domain of Gelatinase A, called 222, using an improved version of a method we previously developed for coating proteins onto cell surfaces (3).
Methods and Materials
We adapted the original method for binding proteins to the surface of MSCs (3) by undertaking the coating step with cells in suspension rather than when coated on tissue culture plastic. After validation of the coating methodology by confocal imaging, 222-coated MSCs were tested for their binding to denatured Type II collagen gelatin or plastic and compared with wild type (uncoated) MSCs as a control. In subsequent preliminary experiments the location of coated and wild type MSCs was tracked after injection into mouse knees, using an antibody to human Lamin A/C.
Results
We were able successfully to coat MSCs with FITC-labelled 222 (Figure 1) and we observed greater binding of 222-coated MSCs to type II collagen gelatin than to plastic, whereas wild type (uncoated) MSCs were found to bind more to plastic than to gelatin (Figure 2). Preliminary cell tracking studies demonstrated the feasibility of tracking human MSCs after their injection into mouse knees.
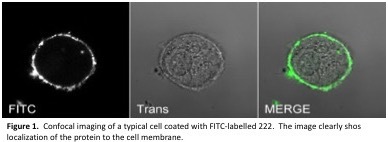

Conclusion
We conclude that MSCs coated with 222 are better able to bind to Type II collagen gelatin and could potentially be used to target injected cells to the cartilage lesion sites.
References
1. A. Vegaet al., Transplantation 99, 1681-1690 (2015).
2. Y. G. Kohet al., Arthroscopy 29, 748-755 (2013).
3. J. P. Armstronget al., Nat Commun 6, 7405 (2015).
18.3.2 - In vivo MRI cell tracking of autologous mesenchymal stem cells in an ovine osteochondral defect model.
- A. El Haj (Birmingham, GB)
- H. Markides (Birmingham, GB)
- K. Newell (Cambridge, GB)
- H. Rudorf (Cambridge, GB)
- L. L. Blokpoel Ferreras (Nottingham, GB)
- J. Dixon (Nottingham, GB)
- M. Graves (Cambridge, GB)
- J. Kaggie (Cambridge, GB)
- F. Henson (Cambridge, GB)
- A. El Haj (Birmingham, GB)
Abstract
Purpose
We aim to devise and implement a SPION (superparamagnetic iron oxide nanoparticle) based technique to non-invasively monitor the intra-articular delivery and bio-distribution of stem cells within a knee joint by MR-imaging. Osteochondral injuries represent a significant clinical problem requiring cell-based therapies to restore function of the damaged joint. Pre-clinical studies are fundamental in translating such therapies, however, technologies to non-invasively assess in vivo cell fate are currently limited. We implement this technique to monitor and compare in vivo cell fate in a pre-clinical ovine model of a chronic and an acute osteochondral injury.
Methods and Materials
Autologous ovine MSCs were isolated, expanded and labelled with Nanomag using the cell-penetrating peptide, P218R. In vitro and ex vivo MRI detection thresholds and cellular viability, proliferation and differentiation were determined prior to in vivo studies. A single 8mm diameter osteochondral defect was created in the medial femoral condyle in the left knee joint of each sheep with the contralateral joint serving as the control. 10x106 Nanomag labelled-MSCs were delivered by intra-articular injection at 1 week or 4.5 weeks post injury. Sheep were sacrificed 7 days post implantation and immediately MR imaged using an ESAOTE 0.2T and Siemens 3T scanner and prior to histological assessement.
Results
In vitro MRI data demonstrated significant increase in MRI contrast as a result of P218R:Nanomag with no impairment of cell function. MRI images revealed evidence of implanted cells within the synovial joint of the injured leg of the chronic model only with no signs of cell localisation to the defect site in either model. This was validated histologically determining the location of implanted cells in the synovium with evidence of engulfment of Nanomag-labelled cells by leukocytes.
Conclusion
We demonstrates the potential of Nanomag and P218R as an effective means of imaging and tracking cells within the knee.
18.3.3 - One-step strategy for cartilage repair using Acellular Bone Matrix scaffold in a preclinical mini-pig model
- L. Dai (Beijing, CN)
- L. Dai (Beijing, CN)
- Y. Ao (Beijing, CN)
- Z. He (Beijing, CN)
- X. Hu (Beijing, CN)
Abstract
Purpose
Cartilage defects are most commonly seen in the knee joint, however, due to the limited self-recovery ability of cartilage, the repair of articular cartilage defects is still a great challenge despite that various approaches were proposed. We designed a strategy to the induction of cartilage repair using ABM (acellular bone matrix) create an appreciate microenvironment for the in-situ cells with easy surgical application.
Methods and Materials
An in vitro system including Scanning electron microscopy (SEM), nanoindentation were used to assess the biomechanical property of the scaffold, while confocal microscopy, and 1,9-dimethylmethylene blue assay were used to assess the adherence, proliferation, and cartilage matrix production of the cells seeded on the scaffold.
Preclinical experiment was carried on a mini-pig cartilage repair model, in which full-thickness defects were produced in the articular cartilage of the trochlear groove of the mini-pig. A total of 36 knee joints of 18 pigs were included and equally divided into 3 groups, undergoing ABM combined with Microfracture (ABM+M group), ABM alone (ABM group), and Microfracture alone (M group). Two pigs (4 knees) in each group were sacrificed at 6, 12, or 24 weeks after the operation, and the repaired tissues were analyzed by histological examination, assessment of matrix staining, SEM, and nanoindentation of biomechanical properties.
Results
The in vitro assessments demonstrated the ABM scaffold could promote cell adhesion, growth and proliferation as well as the chondrogenisis of mesenchymal stem cells. Preclinical experiment in the mini-pig cartilage repair model showed regeneration of hyaline cartilage repair. The biomechanical property of the repaired tissue was similar to that of normal cartilage. The integration of repaired tissue and normal tissue in the ABM+M group were better than other two groups.


Conclusion
This ABM-based, one-stage, minimally-invasive, in situ procedure for cartilage regeneration has the potential to improve the treatment of articular cartilage defects.
18.3.4 - Osteotransduction of an aragonite-based scaffold by human bone marrow-derived mesenchymal stem cells
- K. Zaslav (Richmond, US)
- D. Robinson (PetahTikwa, IL)
- N. Altschuler (Kfar Saba, IL)
- E. Kon (Milano, IT)
- L. Hangody (Budapest, HU)
- K. Zaslav (Richmond, US)
- Á. Berta (Budapest, HU)
Abstract
Purpose
Some aragonite-based scaffolds exhibit osteoconductive properties and are considered useful bone repair scaffolds. The purpose of this study was to evaluate the in vitro osteotransductive potential of the bone phase of a novel aragonite-based bi-phasic osteochondral scaffold (Agili-CTM, CartiHeal Ltd.).
Methods and Materials
Adult human bone-marrow derived mesenchymal stem cells (BM-MSCs) grown in osteogenic medium (Lonza Group Ltd., Basel, Switzerland) were assessed. The study was designed to compare the effect of the bone phase of the Agili-C implant, a coral-derived aragonite scaffold on human BM-MSCs compared to cells grown in an optimal differentiation medium without scaffolds. Analyses were performed at several time intervals: 3, 7, 14, 21, 28- and 42-days post-seeding. Osteogenic differentiation was assessed by morphological characterisation using light microscopy after Alizarin red and von Kossa staining, and scanning electron microscopy. The transcript levels of alkaline phosphatase (ALP), runt-related transcription factor 2 (RUNX2), bone gamma-carboxyglutamate (BGLAP), osteonectin (SPARC) and osteopontin (SPP1) were determined by quantitative PCR. Proliferation was assessed.
Results
Cell cultures of human BM-MSC's demonstrate that the bone phase of the bi-phasic aragonite-based scaffold supports bone formation through enhanced proliferation and osteogenic differentiation of BM-MSCs at both the molecular and histological levels. The scaffold was colonized by differentiating MSCs, suggesting its suitability for incorporation into bone voids to accelerate bone healing, remodelling and regeneration. The mechanism of direct bone formation involves scaffold surface modification with de novo production of calcium phosphate deposits, as revealed by energy dispersive spectroscopy analyses. Scanning the scaffold surface revealed the presence of a microstructure deposit exhibiting a unique morphology (Figure 1) supporting cell attachment (Figure 2).


Conclusion
This implant may promote a fast growth of high-quality bone during the repair of osteochondral lesions. The implant seemed to enhance proliferation of MSCs and formation of multilayer cultures onto its surface.
18.3.5 - Regeneration of hyaline cartilage in joints using minimally invasive fractional laser procedure
- V. Baskov (Moscow, RU)
- V. Baskov (Moscow, RU)
- A. Tokareva (Moscow, RU)
- I. Yaroslavsky (Marlborough, US)
- A. Baskov (Moscow, RU)
- E. Sobol (Marlborough, US)
- A. Shechter (Moscow, RU)
- V. Andreeva (Fryazino, RU)
- A. Kovalenko (Fryazino, RU)
- G. Altshuler (Marlborough, US)
Abstract
Purpose
Investigate Ex-vivo, in an animal study, and in a human clinical study feasibility of using minimally invasive fractional laser procedure to induce cartilage regeneration in patients suffering from osteoarthritis of the knee.
Methods and Materials
Erbium fiber laser emitting at the wavelength of 1560 nm was used in all treatments. Laser energy was delivered to the target site through optical fiber. Ex-vivo, bovine joints were used to investigate temperature rise and tissue damage resulting from laser illumination. In mini-pig animal model, excisional defects with a size of 4–12 mm, depth of 0.5–0.6 mm (partial- thickness lesions) were created on the head of femoral bones (hind legs) of 20 animals. For each animal, an extremity was laser-treated while the contralateral one was left as control. Clinical study was conducted on 12 subjects with osteoarthritis Stage II to III. Laser energy was delivered via surgical arthroscope. All patients received a single treatment. MRI, range of motion, pain, and gait assessments were used for evaluation.
Results
Ex-vivo data has demonstrated that clinically relevant laser parameters induced temperature rise not exceeding 55 ⁰C. It the animal study, majority of the laser-irradiated defects were filled with hyaline-like cartilage in two-three months, in contrast to untreated sites. The restoration of lamina splendens was observed in 60 percent of laser-treated defects. In the clinical study, MRI examinations before and after laser surgery have revealed restoration of the cartilage plate tissue by 1 – 2 mm. For 91% of the patients the results of the dynamic (range of motion) tests have demonstrated essential reduction of the pain syndrome and improved functionality of the joints on 12 months follow-up. 
Conclusion
Pre-clinical and clinical results suggest that minimally invasive fractional laser treatment may be a viable procedure for treating mild-to-moderate osteoarthritis of the knee.
18.3.6 - Therapeutic effects of gefitinib-encapsulated thermosensitive injectable hydrogel in intervertebral disc degeneration
- Z. Pan (Hangzhou, CN)
- Z. Pan (Hangzhou, CN)
- H. Ouyang (Hangzhou, CN)
Abstract
Purpose
Intervertebral disc (IVD) degeneration is one of the most widespread musculoskeletal diseases world- wide, which remains an intractable clinical challenge. The aim of this study is to investigate the thera- peutic potential of the small molecule gefitinib (an epidermal growth factor receptor (EGFR) inhibitor) in ameliorating IVD degeneration.
Methods and Materials
MRI and histological staining were performed to confirm degeneration of the rat and human IVDs. To investigate the functions of EGFR in IVD pathogenesis, we created an inducible Egfr deletion system using Col2a1-CreERT2; Egfrf/f mice. We next evaluated the effect of pharmacological inhibition of EGFR signaling with gefitinib on IVD degeneration. Puncture-induced rat IVD degeneration model was established to evaluate the therapeutic effect of gefitinib-encapsulated ther- mosensitive injectable hydrogel in vivo.
Results
Aberrant EGFR activation levels were detected in both human and rat degenerative IVDs, which prompted us to investigate the functional roles of EGFR by utilizing inducible cartilage-specific EGFR-deficient mice. We demonstrated that conditional EGFR deletion in mice increased nucleus pulposus (NP) extracellular matrix (ECM) production and autophagy marker activation while MMP13 expression decreased. These outcomes are comparable to the use of a controlled-release injectable thermosensitive hydrogel of gefitinib to block EGFR activity in a puncture-induced rat model. We also conducted a case series study involving patients with non-small cell lung cancer and IVD degeneration who received gefitinib treatment from 2010 to 2015. Gefitinib-treated patients displayed a relative slower disc degenerating progression, in contrast to control subjects.


Conclusion
These findings thus provide evidence that suppression of EGFR by the FDA-approved drug gefitinib can protect IVD degeneration in rats, implying the potential application of gefitinib as a small molecule drug for treating IVD degeneration.
18.3.7 - Injectable gels for cartilage repair: a long-term follow-up study in an equine chondral defect model.
- S. Both (Enschede, NL)
- S. Both (Enschede, NL)
- R. Vindas Bolanos (San Jose, CR)
- S. Cockelaere (Utrecht, NL)
- N. Korthagen (Utrecht, NL)
- B. Zoetebier (Enschede, NL)
- P. Dijkstra (Enschede, NL)
- A. Lindahl (Göteborg, SE)
- J. De Grauw (Utrecht, NL)
- R. Van Weeren (Utrecht, NL)
- M. Karperien (Enschede, NL)
Abstract
Purpose
To create an injectable hydrogel that can be used to plaster eroded cartilage surfaces and / or to fill up focal cartilage defects in a minimally invasive arthroscopic procedure. The hydrogel should protect the damaged cartilage surface against further cartilage erosion and possess an optimized environment facilitating cartilage regeneration.
Methods and Materials
We developed an injectable hydrogel by conjugating hydroxyphenyl groups in the backbone of naturally occurring polymers such as dextran and hyaluronic acid, which resemble glycosaminoglycans in the extracellular cartilage matrix. This renders an injectable in situ gelating hydrogel which crosslinks in a peroxidase mediated reaction initiated by non-toxic concentrations of H2O2. In vivo studies were performed in an equine focal chondral defect model with 2 weeks, 3 & 7 months follow up. The hydrogel treatment was compared with nanofracture.
Results
The two week study proved that our hydrogel can be easily applied in a minimal invasive, arthroscopically guided procedure. It also revealed that as early as after two weeks cartilage-like cells migrated into the hydrogel and displayed a chondrocyte like growth pattern (figure 1a). In all studies the horses recovered well from surgery and did now show lameness during the entire studies.
In the 7 months study the differences in cartilage repair between the hydrogel and the nanofracture treatment were assessed with the ICRS-II scoring system (blinded n-3). The hydrogel treated joint showed significantly better scores for every ICRS-II criterion except matrix staining with an overall ICRSii score of 72% versus 48%. Defects treated with nanofracture contained mainly fibrous tissue and fibrocartilaginous repair tissue (figure2). Three months after the start one horse died, due to circumstances not related to the experiment, cartilage repair was observed (figure1b).


Conclusion
The clinical and histological outcome of these studies suggest that this hydrogel could provide a clinical effective treatment for the cell-free repair of focal cartilage defects.
18.3.8 - Ovine knee cartilage differs in structural and functional properties across articular surfaces
- B. Nelson (Fort Collins, US)
- B. Nelson (Fort Collins, US)
- M. Risch (Fort Collins, US)
- J. Easley (Fort Collins, US)
- E. McCready (Fort Collins, US)
- K. McGilvray (Fort Collins, US)
- K. Troyer (Fort Collins, US)
- J. Johnson (Fort Collins, US)
- J. Kisiday (Fort Collins, US)
Abstract
Purpose
Articular cartilage is a critical joint tissue. Once damaged, it has limited repair potential and large efforts have gone towards investigating emerging repair strategies. Sheep are commonly used because of their ability to translate results to human orthopedic conditions, although it is not known if variation in structural and functional properties exist across the surface. Therefore, the purpose of this study is to characterize cartilage heterogeneity as a function of location in adult ovine stifles by measuring mechanical performance (creep behavior), biochemical properties (GAG content), and thickness across the femoral condylar and trochlear surfaces.
Methods and Materials
In ten stifle (knee) joints, cartilage regions of interest (ROIs) were generated within each femoral trochlea (8 ROIs) and condyle (6 medial, 6 lateral). Each cartilage ROI was evaluated with macroindentation testing, dimethylmethylene blue assay and µCT scan to determine percent creep, GAG content and cartilage thickness, respectively. Results of each variable were mapped based upon anatomical locations and compared using a mixed model ANOVA. Significance was defined as P<0.05.
Results
For percent creep (Figure 1), ROIs within each knee location were significantly different (lateral condyle: P<0.0001; medial condyle: P<0.0001; trochlea: P=0.0004). Percent creep on the medial femoral condyle was significantly lower than the femoral trochlea and lateral condyle (both P<0.0001). For GAG content (Figure 2), there were significant differences between ROIs within the following knee locations: lateral condyle (P<0.0001), medial condyle (P=0.01) and trochlea (P<0.0001). When evaluated collectively, differences in GAG content between knee locations were not detected. Medial femoral condyle cartilage was significantly thicker than the femoral trochlea and lateral condyle (P<0.0001).


Conclusion
Cartilage mechanical properties, biochemical composition and thickness vary across distal femoral joint surfaces. Clinicians and researchers working with ovine cartilage should recognize this potential variability and standardize cartilage sites accordingly to obtain accurate results based on study goals.
18.3.9 - The results of cartilage damage treatment using growth plate chondrocytes on the growing animal model.
- R. Tomaszewski (Katowice, PL)
- R. Tomaszewski (Katowice, PL)
- Ł. Wiktor (Chorzów, PL)
Abstract
Purpose
Due to the specific structure and highly specialized function, treatment of articular cartilage damage is a major clinical problem. Moreover its avascular character results in low regenerative capacity. The aim was to compare the results of treatment of cartilage damage treated using scaffold , bone marrow, and growth plate chondrocytes.
Methods and Materials
The study group consisted of 10 pigs at the age of 12 weeks. a bone marrow biopsy was performed from the posterior iliac spine. We divided the scaffolds into two groups. First one consisted scaffold with bone marrow and growth plate chondrocytes. Second group contended only of scaffolds with bone marrow without chondrocytes . The samples from pig’s knee according to ICRS classifications was evaluated after 12 weeks from the day of surgery.
Results
Macroscopic evaluation according to ICRS classification, in the group of pigs treated with scaffolds without chondrocytes, all animals showed nearly normal morphology with an average of 9.66/ 12 points. In the group of pigs treated with scaffolds incubated with chondrocytes, all animals similarly showed nearly normal morphology with an average of 10.44/ 12 points. Based on microscopic evaluation, 1 very good results and 8 good results were obtained in the group of pigs treated with scaffolds without cells, with an average of 70.44%; in the group of pigs treated with the scaffolds incubated with the cells, 5 very good results and 4 good results were obtained with an average of 79.61%.
Conclusion
1. Treatment of joint cartilage damage with the use of additional growth cartilage chondrocytes improves the quality of joint cartilage healing process.
2. Chondrocyte clustering is a manifestation of the cartilage repair process starting from the side of the stimulated subchondral bone.
18.3.10 - Osteochondritis Dissecans Chondrocytes: A Novel Source for Cartilage Tissue Engineering
- L. Moncada-Palazuelos (Davis, US)
- L. Moncada-Palazuelos (Davis, US)
- V. Krasnodemsky (Davis, US)
- O. Sayfan (Davis, US)
- A. Kapatkin (Davis, US)
- B. Filliquist (Davis, US)
- P. Chou (Davis, US)
- D. Marcellin-Little (Davis, US)
- N. Vapniarski-Arzi (Davis, US)
Abstract
Purpose
Osteochondritis dissecans (OCD) is the result of abnormal development of cartilage leading to joint disease across many species including dogs. Surgical treatment is palliative and includes removal of the detached cartilage fragment. These fragments contain viable chondrocytes and can serve as a unique cell-source for cartilage tissue engineering. The purpose of this study is to prove that a novel scaffold-free approach can yield neocartilage tissue with properties akin to native cartilage.
Methods and Materials

OCD fragments are enzymatically digested and matrix-free chondrocytes are expanded for 2-3 passages. The cells are then transferred onto a no-adherent bioreactor suspension culture for 10 days where cells form spherical aggregates. Single cells obtained by enzymatic digestion of aggregates are then seeded at high density into custom-made agarose molds where a process of self-assembling materializes. In this process, chondrocytes coalesce and generate tissue-specific extracellular matrices (ECM) such as glycosaminoglycans and collagen. During the construct maturation period of 4 weeks, both mechanical and biological stimuli are introduced in a consecutive, simultaneous and synergistic fashion. Constructs are collected on day 28 of seeding and subjected to mechanical and biochemical testing.
Results

Our methods yielded dozens of 0.8x1.4cm cartilage constructs from one 0.3mm in diameter OCD fragment. The biomimetic index that reflects the overall mechanical and biochemical properties of the engineered construct relative to native tissue was 88%. The compressive and tensile stiffness of engineered cartilage was 501kPa and 2.3MPa, respectively. The glycosaminoglycan content was 26.4%/DW and collagen 6.0%/DW.
Conclusion
The high degree of biomimicry achieved by this study without the use of exogenous scaffolds holds promise for clinical translation. The custom-made agarose molds that define the shape and size of these scaffold-free constructs can be tailored to individual patient needs using 3D printing technology. Lastly, demonstrating the feasibility of this approach in a naturally-occurring canine disease-model offers an additional translational advantage.