The program times are listed in Central European Time (CEST)
THE DESIGN AND EVALUATION OF AN AUTOMATIC PRANDIAL INSULIN DOSING SYSTEM FOCUSED ON SAFETY
Abstract
Background and Aims
We designed a safety-focused methodology for automatically dosing prandial insulin by utilizing a glycemic disturbance detector.
Methods
By using regularized deconvolution to solve for unknown disturbances, we simulated how changes in insulin doses would affect glycemia in a clinical dataset of 14 patients over a month (ClinicalTrials.gov NCT03859401). Our automatic bolus strategy relied on an algorithm that determined the probability of glycemic disturbances requiring insulin to prevent hyperglycemia. Percentages of the individual’s total daily insulin (TDI) were delivered at different probability thresholds. After injection, blood glucose was simulated for 120 minutes and the number of hypoglycemic events was counted. We determined an escalating dosing strategy based on what led to no more than one additional hypoglycemic event per day. We then simulated the dataset using automatic doses to augment or replace boluses in the record.
Results
From our criteria, we chose thresholds of 3%, 4%, 5%, 6%, and 9% TDI at probabilities of 0.3, 0.5, 0.7, 0.8, and 0.9, respectively. When the full dataset was simulated, there was an increase of 0.88% percent time <70 mg/dL, a 2.85% increase in percent time 70-140 mg/dL, a 2.09% increase in percent time 70-180 mg/dL, a reduction of 2.97% percent time >180 mg/dL, and 1.47% less percent time >250 mg/dL when compared to the observed hybrid closed-loop (HCL) data.

Figure 1 - Number of hypoglycemic events per day at different TDI percentages and thresholds.
Conclusions
This automatic bolusing strategy increased euglycemia and decreased hyperglycemia in simulation when compared to HCL, while only increasing hypoglycemia slightly.
REAL-WORLD OUTCOMES OF THE FIRST 1’000 USERS OF THE MINIMEDTM 780G SYSTEM
Abstract
Background and Aims
The MiniMedTM 780G system clinical trials demonstrated improved glycemic control of >70% of time spent in target glucose range (70-180 mg/dL, TIR) and HbA1c or Glucose Management Indicator (GMI) of <7%.1,2 Following introduction of the system in Europe, in October 2020, real-world glycemic control outcomes were evaluated.
Methods
MiniMed™ 780G system data from countries having ≥50 users were uploaded voluntarily to CareLinkTM Personal software from 05October2020- 11December2020 by individuals providing consent, and analyzed. Mean sensor glucose (SG), GMI, and percentage of time spent across SG ranges and in closed loop (automated basal, at minimum) were determined for users having ≥10 days of SG data after initiating MiniMed™ 780G automated basal and correction boluses.
Results
Individuals (N=1033) had a TIR of 76.8±9.1% and SG of 142.6±14.3 mg/dL (7.9 mmol/L), corresponding to a GMI of 6.7±0.3% (Figure). Time spent at <70 mg/dL and <54 mg/dL was 2.7±2.3% and 0.6±0.9%, respectively, while time spent at >180 mg/dL was 20.4±9.3%. The percentage of users achieving a TIR >70% and a GMI <7.0% was 80.2% and 82.4%, respectively. Time below ranges of <1% (for <54 mg/dL) and <4% (for <70 mg/dL) were achieved by 74.8% of users, and while in closed loop 92.7±10.8% of the time.
Conclusions
In this first analysis of real-world MiniMedTM 780G system data, most users achieved internationally recommended goals of glycemic control for TIR, TBR3 , and GMI. These results are similar to those observed in the MiniMedTM 780G system clinical trials, supporting real-world reproducibility and providing evidence to the robustness of the algorithm.

ATTD CONSENSUS TARGETS ARE ACHIEVABLE FOR PRESCHOOLERS: HYBRID CLOSED LOOP USE IN CHILDREN FROM 2-14 YEARS
Abstract
Background and Aims
Currently, only one CE marked systems with a hybrid automated insulin delivery (H-AID) is available per prescription in Germany. It is not labelled for use in preschoolers. The only technical limitation of this system (Minimed670G) for small children is a daily use of 8 units of insulin. ATTD consensus targets recommend a Time in Range (TIR) of >70% and Time below Range <4% in all ages with T1D.
Methods
A two phase study of children aged 7-14 and <7 years was conducted at our center. All children received training for the system and used it for 8 weeks in manual mode or 8 weeks in auto mode, the order was randomly assigned. Primary outcome parameter was the TIR 70-180%.
Results
 20 children from 2.5 to 6 years and 17 from 8-14 years completed the trial; younger group recruitment was stopped preliminary by Covid19 pandemic. After 8 weeks of HCL, TIR in 14day-profile was 70.7 % compared to 53.8% after PLGM in the older group, HCL 73.9 % vs. PLGM 68.2% in preschool kids. Details on achievement are shown in table.
20 children from 2.5 to 6 years and 17 from 8-14 years completed the trial; younger group recruitment was stopped preliminary by Covid19 pandemic. After 8 weeks of HCL, TIR in 14day-profile was 70.7 % compared to 53.8% after PLGM in the older group, HCL 73.9 % vs. PLGM 68.2% in preschool kids. Details on achievement are shown in table.
Conclusions
As shown in other trials, 670G is safe even under the labelled age. TIR was higher in our study than published before. In both groups best results were achieved with the H-AID system. Prescoolers benefit from their parents performing the insulin therapy. To ensure safe use and modalities for children and prescribers, a label is also needed for small children.
VIRTUAL CAMP EFFECTIVENESS TO START NEW TECHNOLOGIES IN FAMILIES WITH CHILDREN LIVING WITH TYPE 1 DIABETES
- Valentino Cherubini, Italy
- Rosaria Gesuita, Italy
- Riccardo Bonfanti, Italy
- Sara Giorda, Italy
- Lorenzo Lenzi, Italy
- Claudio Maffeis, Italy
- Marco Marigliano, Italy
- Monica Marino, Italy
- Ivana Rabbone, Italy
- Riccardo Schiaffini, Italy
- Sonia Toni, Italy
- Andrea Rigamonti, Italy
- Barbara Piccini, Italy
- Andrea E. Scaramuzza, Italy
- Virtual Camp Study Group, Italy
Abstract
Background and Aims
To analyze the impact of an educational Virtual Camp (eVC) in children with T1D and their parents on glucose control.
Methods
Nineteen Italian centers for pediatric diabetes participated. The eVC was held from 6 to 8 November 2020; it involved parents and their children aged 6-17 years who previously updated their pump software from Tandem Basal-IQ to Control-IQ technology. Several interactive sessions involving pediatric diabetologists, psychologists, dieticians and physical education teachers have been performed during the eVC. Eight weeks before the update to Control-IQ and eight weeks after eVC, CGM metrics were downloaded and summarized using median and interquartile range (IQR) and compared by Wilcoxon sign-rank test. Median differences and 95%CI were estimated.
Results
43 children, 53.5% females, median age 15y and diabetes duration 10y, were included. After the eVC, the percentage of time in range and time above range significantly improved (Table 1); %CV and %GMI significantly decreased; IQR values showed a lower interindividual variability when using the updated algorithm. In addition, a significant reduction of daily percentage of bolus insulin was reported.

Conclusions
A significant huge improvement of glucose metrics was observed in children with T1D using Control-IQ technology after a 3-day eVC. More than 75% of children maintained a percentage of TIR over 70% during an 8-week period after eVC, suggesting that, despite forced interruption of clinical visits due to Covid-19 pandemic, the eVC is a useful tool to start new technological devices.
3-MONTH EVALUATION OF ADVANCED HYBRID CLOSED-LOOP IN CHILDREN AND ADOLESCENTS WITH TYPE 1 DIABETES: A SINGLE-CENTER EXPERIENCE IN ITALY
Abstract
Background and Aims
The Medtronic Minimed® Advanced Hybrid Closed-Loop system (AHCL) includes an individualised algorithm with optional set points, automated correction bolus, and improved SmartGuard™ Auto Mode stability. After clearing the EU CE Mark in June 2020, it was in the market in Italy starting first week of October 2020. We present the first 3-month results of AHCL in children and adolescents with type 1 diabetes from a single center.
Methods
This one-centre, user-evaluation study in 25 children, adolescents and young adults (aged 1-25yrs), compared AHCL (MiniMed 780G, Medtronic, Northridge, CA, US) automated mode to first 14 days using manual mode, similar to Predictive Low Glucose Management (SAP+PLGM). Time in range (TIR), above range (TAR), below range (TBR), coefficient of variation (CV), mean sensor glucose, GMI, and severe adverse events (severe hypoglicemia and diabetic ketoacidosis in auto vs manual mode are presented.using median and interquartile range (IQR) and compared by Wilcoxon sign-rank test. Median differences and 95%CI were estimated.
Results
Twenty-five patients, 70% males, median age 14y and diabetes duration 11y, were included. Nineteen switched from a hybrid closed-loop system (MimiMed 670G), 4 from multiple daily injections and 2 from other insulin pumps. After 3-month using AHCL, TIR significantly improved and TAR decreased, while TBR did not change; %CV and %GMI significantly decreased (Table).

Conclusions
AHCL (MiniMed 780G system) with automated correction bolus showed a significant improvement in glucose metrics compared to PLGM (manual mode of the system), in children, adolescents and young adults with type 1 diabetes using AHCL after a 3-month usage.
NEW PROPOSED PHYSIOLOGY MODELS THAT UTILIZE METABOLIC EXPENDITURE DATA FROM ACTIVITY SENSORS TO FORECAST CHANGES IN GLUCOSE DURING AEROBIC EXERCISE
Abstract
Background and Aims
People with type 1 diabetes can experience dramatic changes in glucose during aerobic exercise leading to hypoglycemia. Physiological models of glucose dynamics do not correctly estimate glucose changes during exercise. This project compares two candidate mathematical model structures of glucose metabolism during exercise. Both models include metabolic expenditure as an input derived from body-worn physical activity sensors.
Methods
Models were identified and evaluated using a secondary analysis of data collected from adults with T1D who participated in a glucose-tracer infusion study (n = 17, 11 F, weight 78.2 ± 11.0, TDIR 56.2 ± 12.7). After randomization into moderate intensity (40-45% V02 max) vs. high intensity (60-65% V02 max) group, participants performed in-clinic exercise on three days clamped at infusions of low (basal), medium (1.5x basal), or high (3x basal) insulin rate, and performed 45 minutes of aerobic exercise. Models of exercise impacted endogenous glucose production (EGP), and rate of glucose disposal (Rd). Model 1 was designed such that EGP and Rd from exercise were not dependent on insulin. Model 2 included both an insulin-dependent and non-insulin-dependent component of exercise-induced EGP and Rd. Parameters for Models 1 and 2 were fit using a Hamiltonian Monte-Carlo sampling scheme.
Results
Model 1 was more accurate than model 2 when evaluated across all study data (root-mean-squared error 0.71±0.20 mmol/L Vs. 1.10±0.35 mmol/L), and in estimating glucose changes during exercise (RMSE 0.97±0.77 mmol/L Vs 1.22±0.81 mmol/L).
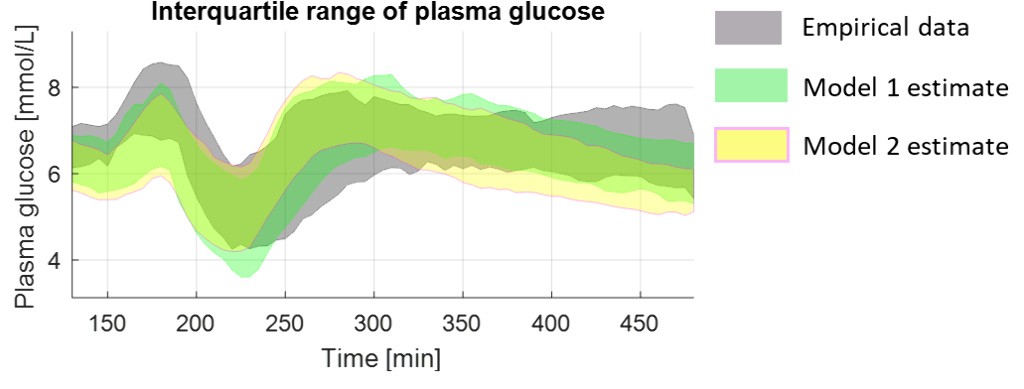
Conclusions
Additional models will be proposed and evaluated prior to incorporation into existing virtual patient populations and model predictive control closed-loop algorithms.
SWITCHING FROM BASAL-IQ TO CONTROL-IQ TECHNOLOGY IN CHILDREN AND ADOLESCENTS WITH TYPE 1 DIABETES: ONE WEEK IS ENOUGH TO IMPROVE TIME IN RANGE.
- Marco Marigliano, Italy
- Andrea E. Scaramuzza, Italy
- Riccardo Bonfanti, Italy
- Rosaria Gesuita, Italy
- Sara Giorda, Italy
- Lorenzo Lenzi, Italy
- Monica Marino, Italy
- Barbara Piccini, Italy
- Ivana Rabbone, Italy
- Andrea Rigamonti, Italy
- Riccardo Schiaffini, Italy
- Sonia Toni, Italy
- Claudio Maffeis, Italy
- Valentino Cherubini, Italy
- Virtual Camp Study Group, Italy
Abstract
Background and Aims
In people with Type 1 Diabetes (T1D) the percentage of time in Range (TIR), 70-180 mg/dL, is now recognized as the most effective glucometrics together with HbA1c. The aim of this study was to analyze the early effect on TIR changes after switching from Tandem Basal-IQ to Control-IQ technology in a group of children and adolescents with T1D.
Methods
Children and adolescents from 19 Italian centers have been recruited. After a standard educational program, the enrolled patients updated pumps software from Basal-IQ to Control-IQ. Differences in TIR one week (excluding the day of the update) and three weeks before and after the update were analyzed. TIR values were summarized using median and interquartile range (IQR) and compared by Wilcoxon sign-rank test. Median differences in TIR and 95%CI were used.
Results
TIR data of 43 youths (53.5% females, median age 15y, diabetes duration 10y) were analyzed. After upgrading to Control-IQ technology, TIR significantly improved, starting after the first week [median (IQR)], 75% (70;82) vs 67% (53;73), p<0.001, and keeping steady for the whole 3-week observation: 76% (69;82) vs 65% (55;73), p<0.001 (Figure 1). Furthermore, with Control-IQ algorithm, there was a lower interindividual variability as shown by the reduced differences in the IQR.
Conclusions
A significant increase in TIR is already evident after the first week with Tandem t:slim Control-IQ technology and this improvement is kept in the following weeks of observation.
COMPARING USE OF THE OMNIPOD® 5 SYSTEM WITH 3 MONTHS OF AUTOMATED INSULIN DELIVERY TO 3 MONTHS IN MANUAL MODE: A POST-HOC CROSSOVER ANALYSIS
- Bruce A. Buckingham, United States of America
- Lisa Norlander, United States of America
- Irl Hirsch, United States of America
- Lauren Huyett, United States of America
- Todd Vienneau, United States of America
- Steven Lowen, United States of America
- Bonnie Dumais, United States of America
- Trang Ly, United States of America
Abstract
Background and Aims
Achieving treatment goals can be challenging for people with type 1 diabetes (T1D) due to the many daily manual tasks and decisions required. During a 3-month single-arm pivotal study of the Omnipod 5 automated insulin delivery system, a 3-month mid-study safety pause provided the opportunity to assess the system in manual mode (MM, algorithm inactive) compared to automated mode (AM, algorithm active) in a post-hoc crossover analysis.
Methods
A subset of adults (14-70y) and children (6-13.9y) with T1D participating in the study completed four distinct phases of therapy: (1) run-in phase with their standard therapy (ST, 14d), followed by use of the Omnipod 5 System in (2) AM, (3) MM (3mo.), and (4) AM. The total duration of AM use was 3 months per participant (phases 2+4 combined). The primary glucose outcome was percent time in range (TIR, 70-180mg/dL, 3.9-10.0mmol/L).
Results
Adults (N=83) and children (N=89) were aged (mean±SD) 36±14y and 10.5±2.1y with T1D duration 16±11y and 4.8±2.7y and baseline A1C 7.3±0.9% and 7.7±0.9%, respectively. TIR was significantly higher during the AM phases compared to ST and MM (Table). Specifically, TIR increased with AM during Phase 2 compared to ST, reduced back to a level comparable to ST during MM in Phase 3, and then increased again when AM resumed in Phase 4 (all p<0.05).
Conclusions
This post-hoc crossover study emphasizes the effectiveness of the Omnipod 5 automated insulin delivery algorithm on glycemic outcomes, beyond pump and CGM use alone.

DIABELOOP DBL4K HYBRID CLOSED-LOOP SYSTEM IMPROVES TIME IN RANGE WITHOUT INCREASING TIME IN HYPOGLYCEMIA IN CHILDREN AGED 6-12 YEARS.
Abstract
Background and Aims
The DBLG1 Hybrid Closed-Loop system improves time in range and glycemic control in adults. However, efficacy and safety of the system had not yet been evaluated in children.
The objective is to evaluate the non-inferiority of the DBL4K (Diabeloop for Kids) hybrid closed-loop system (Kaleido pump + Dexcom G6 = CL) compared to a Dexcom G6 sensor augmented pump (open loop = OL) in prepubescent children.
Methods
Multicenter open-label randomised controlled trial in 3 pediatric diabetology centers. The CL and OL are worn for 4 days in hospital, followed by 6 weeks at home.
Results
Twenty-one patients (mean age: 8.3+/- 1.6 years; 10 boys) were included between March and December 2019. The percentage of time spent in range (4-10 mmol/L) was identical in both groups during the 72-hour phase in hospital (68.73% in CL vs 70.53% in OL, p=0.539) then significantly higher in the CL group during the 6 weeks at home (66.19% in CL vs 58.68% in OL, p<0.001). The percentage of time spent in hypoglycemia (< 3.85 mmol/L) was significantly lower in the CL group during the 72 hours in hospital (2.04% in CL vs 7.06% in OL, p<0.001) and the 6 weeks at home (2.62% in CL vs 5.24% in OL, p<0.0001). No events of ketoacidosis or severe hypoglycemia were recorded.
Conclusions
The DBL4K Closed-Loop System is suitable for prepubescent children. Just as in adults it improves the time in range while decreasing the time spent in hypoglycemia. It could allow for better long-term glucose control in children with diabetes.
PHYSICIANS' ATTITUDES TOWARD AID-SYSTEMS AND THEIR IMPLICATIONS FOR DIABETES CARE
Abstract
Background and Aims
To date, there has been little research on the impact of AID systems in routine diabetes care.
Methods
337 diabetologists (2018: 422; 2019: 324; 43% female, average age 53.2 years) were asked about their attitudes and expectations for AID systems.
Results
59.4% of physicians currently consider AID systems to be very important for people with diabetes, and 91.6% will do so in 5 years. The majority of diabetologists expect that diabetes care will become more complex with AID systems (85.2 %), that more education is needed (81.4%) to facilitate the use of AID systems and will also result in an increase in the amount of time spent with the patient (65,9%). 48.3% believe that diabetes patients with AID systems will become more autonomous and be able to manage their diabetes more independently. 41.4% agree with the statement that many patients will not be able to manage AID systems in the right way. However, only 13.9% also share the view, that AID systems will make diabetes therapy riskier. Overall, the diabetologists do not believe that AID systems result in fewer contacts with patients (21.8 % agreement), less contact with patients (21.8% agreement), reduced amount of care required (13.9 % agreement) or that the diabetes team will become even become superfluous (3.5% agreement).
Conclusions
Overall, diabetologist estimate AID systems as a very important innovation. The effort required for education, therapy adjustment and further support is considered to be relatively high. There are less fear that diabetes team will become less important.
