James M. Richardson, Switzerland
Ascensia Diabetes Care Medical AffairsPresenter of 2 Presentations
INTERFERENCE EVALUATION OF THE CONTOUR®CARE TEST STRIP
Abstract
Background and Aims
Blood glucose monitoring systems (BGMSs) have the potential to provide inaccurate results in the presence of interfering substances found in the blood resulting in several serious and some fatal events being documented. Furthermore, some conditions such as anemia, cancers, renal disease, dietary deficiencies, rheumatoid arthritis and during pregnancy may affect concentrations of endogenous interfering substances. The effects of interfering substances is of clinical relevance during BGMS selection.
A new BGMS platform utilizing the CONTOUR®CARE test strip encompassing the FAD-GDH enzyme was assessed for common endogenous and exogenous substances for interference effect in accordance with ISO 15197:2013 and FDA guidelines.
Methods
Interfering substances were tested in pooled venous blood at glucose concentrations of 60 mg/dL, 120 mg/dL and 260 mg/dL with guidance from the Interference Testing in ISO 15197:2013 and FDA guidelines. Each interferent was assessed at each of the three glucose concentrations with three test strip lots in ten meters resulting in 90 measurements per interferent.
Results
Results were analyzed according to ISO 15197:2013 Section 6.4.4 guidelines (interference effects shall be described in the instructions for use if the average difference between test and control samples exceeds 10 mg/dL [0.55 mmol/L] or 10% at glucose concentrations < 100 mg/dL [5.55 mmol/L] and ≥ 100 mg/dL [5.55 mmol/L], respectively). All of the interfering substances passed with the exception of xylose.
Conclusions
All of the assessed interfering substances passed the acceptance criteria with the exception of xylose which is no longer commonly used in clinical practice.
CHALLENGES OF HYPOGLYCEMIA MANAGEMENT USING MOBILE APPLICATIONS
Abstract
Background and Aims
Many mobile applications (apps) for type 1 and 2 diabetes management incorporate results from BGMSs, however there is a paucity of evidence demonstrating benefits of apps for hypoglycemia management. Hypoglycemia management is associated with the performance of BGMSs in low blood glucose range (LBGR) and any app reliant on BG results is subsequently dependent. Previously we presented data demonstrating a reduced risk of hypoglycemia associated with the use of the CONTOUR®DIABETES app (CDA) whilst utilizing CONTOUR®NEXT ONE BGMS (CNO). Here we study the possible variation of hypoglycemia detection if BGMSs other than CNO were used with the app to obtain BG readings
Methods
All BGMSs were assessed for the likelihood of results ±15% of reference BG value of 50 mg/dl (CI 95%). We assumed that all assessed BGMSs were used with the app and all BG readings were analyzed identically as it has been performed in the CDA study using CNO.
Results
The probability of achieving ±15% of reference BG value of 50 mg/dl of some BGMSs were below 95% (Figure 1) and LL of BG readings of one BGMS (ACAC) were >50 mg/dl.
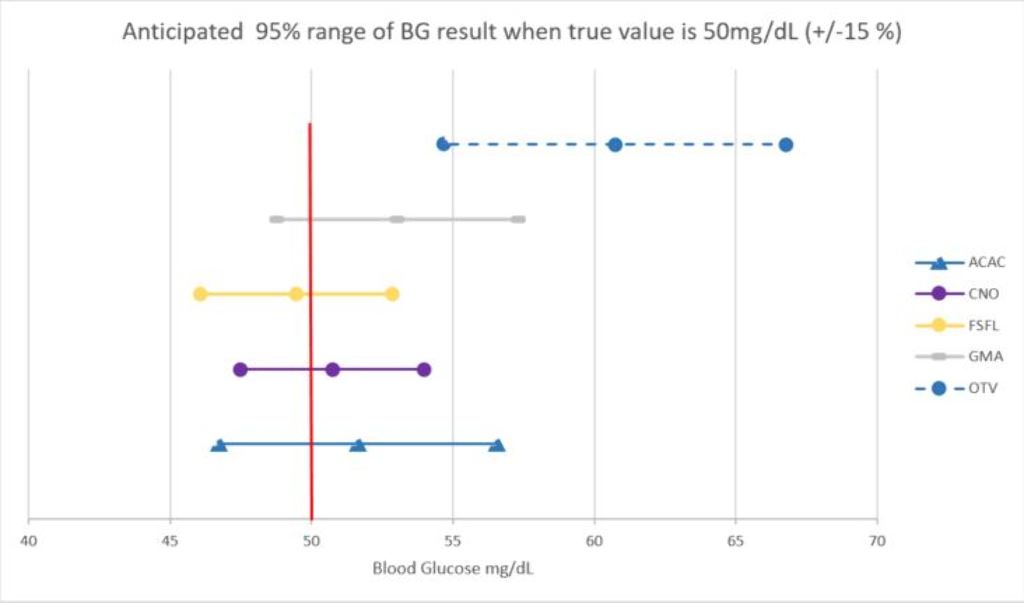
Conclusions
Not all BGMSs utilizing apps are capable of detecting hypoglycemia with a high probability thus not leading to potential reduced hypoglycemia occurrence as previously demonstrated. This analysis demonstrates that the selection of BGMS is critical in assessing the effectiveness of apps on glycemic control and particularly hypoglycemic management.
