ARISES: AN ADVANCED CLINICAL DECISION SUPPORT PLATFORM FOR THE MANAGEMENT OF TYPE 1 DIABETES
Abstract
Background and Aims
ARISES (Adaptive, Real-time, Intelligent System to Enhance Self-care of chronic disease) is a mobile platform facilitating a decision support system for people with Type 1 diabetes (T1D). This project aims to improve the efficacy of current care and reduce the burden of managing diabetes in daily living. We incorporate wearable biosensors and leverage on-device machine learning techniques towards improving glucose control.
Methods
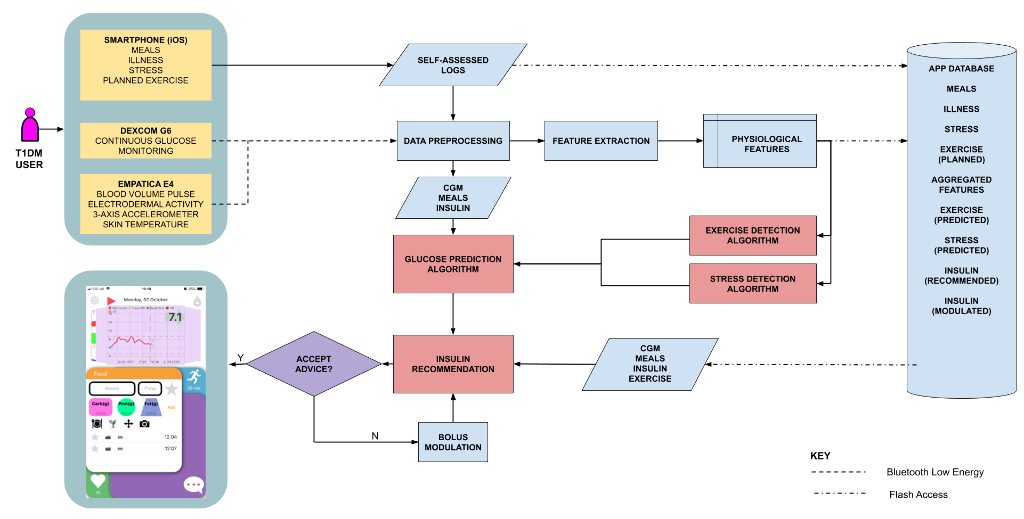
This work details the system architecture for ARISES. ARISES comprises inputs from a Dexcom G6 for CGM, and Empatica E4 for physiological signals. These data are wirelessly transmitted to the smartphone. These data are then input to machine learning models which provide insights on the future glucose trajectory by means of a dilated recurrent neural network algorithm, and detect external contributing factors – exercise and stress. The second component is an insulin recommendation algorithm based on deep reinforcement learning that, using insights and historical data, provides bolus advice.
Results
We developed the platform on Apple iOS and implemented it on an iPhone. The built-in algorithm has been in silico validated and evaluated with retrospective clinical data. The performance of the platform will be evaluated in a randomised cross-over clinical study with 12 participants over 8 weeks.
Conclusions
The ARISES platform represents a step forward in decision support systems for T1D management due to the wealth of collected physiological data and the use of state-of-the-art machine learning algorithms.
A NOVEL HAND-HELD INTERFACE SUPPORTING THE SELF-MANAGEMENT OF TYPE 1 DIABETES
Abstract
Background and Aims
The novel ARISES app designed to be used by people with Type-1 diabetes incorporates a number of interface features designed to enhance its usability. One feature is a diary offering both focus-plus-context and historical views of medical data. Another is the novel use of context (Figure). A third is the smooth manual exploration (“What if . . ?”) of the effect of carbohydrate intake on predicted blood glucose level and recommended insulin dose.
Methods
Usability evaluation is of paramount importance and will take two forms. One is a full conventional clinical trial in the course of which a number of quantitative and qualitative usability outcomes will be observed. That will follow a pilot study to identify possible improvements. For this latter study, a novel concept called Circles of Affordance has been devised. It is economic and designed to show a user’s departure from optimum behaviour.
Results
Implementation of the interface is at an advanced stage and will be complete by November 2019. The Circles of Affordance diagram has been through initial tests based on a number of virtual scenarios.

Conclusions
Design of the smartphone app's interface has benefitted from feedback from both representative users and interface design specialists, and its implementation is almost complete. Plans are in place to carry out usability studies.
IMPLEMENTATION OF CDISC CLINICAL DATA STANDARDS FOR TYPE 1 DIABETES (T1D) TO PROMOTE DATA SHARING AND REUSE
Abstract
Background and Aims
Adoption of clinical data standards can transform otherwise incompatible and disparate data into useful and interoperable information that can enable impactful discoveries for T1D and clinical research more broadly. This project is developing T1D data standards for Pediatrics, Devices, Exercise and prevention data.
Methods
Following the CDISC consensus-based, clinical data standards process and with support from T1D clinical and standards development experts, the current CDISC diabetes standards are being expanded. The CDISC standards development process consists of five stages:
1) Scoping
2) Concept Modeling
3) Standards Development
4) Internal Review
5) Public Review
6) Publication
Results
September 2019 the T1D data standards for Pediatrics & Devices (P&D) became available for Public Review and will be published by end of Q12020 for the use of researchers and industry. The first implementation of the P&D is expected to be the T1DEXI observational study data that will be made available publicly.
T1D data standards for Exercise and Prevention (E&P) will be available for Public Review in Q12020. We encourage all ATTD attendees to contribute!
Conclusions
Required by US FDA and Japan PMDA for all drug submissions, CDISC standards enable the FAIR (findable, accessible, interoperable, reusable) data practices to be implemented by industry and academia. With the use of data standards, we can overcome the hindrance of “silo-ed” research data to find innovative solutions to diabetes treatments and therapies. CDISC’s vision is to expand the use of data standards to leverage and realize the full potential of research and clinical data.
PREDICTIVE FACTORS CONTRIBUTING TO GLUCOSE CHANGES DURING AEROBIC, RESISTANCE, AND HIGH INTENSITY INTERVAL TRAINING IN TYPE 1 DIABETES
- Peter G. Jacobs, United States of America
- Zoey Li, United States of America
- Gavin Young, United States of America
- Peter Calhoun, United States of America
- Robin L. Gal, United States of America
- Roy W. Beck, United States of America
- Jessica Castle, United States of America
- Mark A. Clements, United States of America
- Eyal Dassau, United States of America
- Francis Doyle III, United States of America
- Melanie Gillingham, United States of America
- Corby Martin, United States of America
- Michael R. Rickels, United States of America
- Susana Patton, United States of America
- Michael C. Riddell, Canada
Abstract
Background and Aims
People with type 1 diabetes (T1D) have difficulty with glucose control during exercise. Exercise type and other factors may have different impact on glycemic control. We used linear mixed effects modelling to identify physiologic features most predictive of changes in glucose during aerobic, resistance and high intensity interval training (HIIT).
Methods
Thirty seven potential predictive factors including glucose from continuous glucose monitoring, insulin, physical activity and food data collected during a 4-week free-living pilot study from 44 people with T1D (age 35±15 years, BMI 26.2±3.1 kg/m2, 19±13 years since diagnosis), were evaluated. Participants using multiple daily injections (n=9) or an insulin pump (n=35) were randomized to complete one of three 30-minute exercise videos twice each week (aerobic [n=19], resistance [n=14], or HIIT [n=11]). Completed exercise included 138 aerobic, 83 HIIT, and 82 resistance sessions. Change in glucose during exercise was calculated as the pre-exercise glucose value minus nadir glucose during exercise.
Results
Results showed that higher mean glucose 1 hour before (P<0.001) and lower mean glucose 24 hours before exercise (P=0.09) were associated with a greater drop in glucose during exercise. Higher insulin on board was also associated with a greater drop during exercise, but was not statistically significant after multiplicity adjustment (P=0.34).
Conclusions
Predictive features identified, including pre-exercise glucose level and insulin on board, may help inform new machine learning algorithms to better protect against hypoglycemia during physical activity.

